 Gas Balloons
Gas Balloons
Gas is ubiquitous. The atmosphere that surrounds us is composed of a thick layer of gases.
Gas molecules are very loosely bound to each other and consequently, are free to move in all directions. Unlike solids, gas has no definite shape or volume. They can fill any volume as their particles diffuse everywhere.
Gas can be compressed easily with very little pressure because they have high intermolecular spaces. Soda can, cylinders, balloons, balls, tires and spray bottles are the few examples in which compressed gas is used.
Gas particles have electrostatic forces that bind them. Like charged areas repel each other, whereas unlike charged areas attract each other. Plasmas are the gases that contain permanently charged ions.
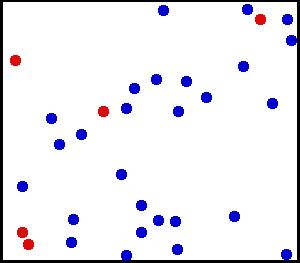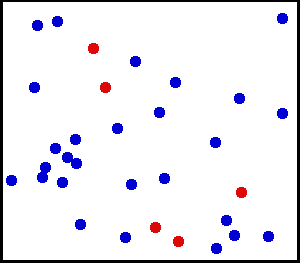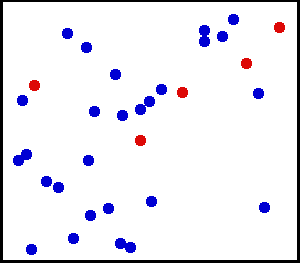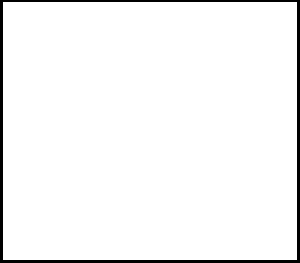 Random collisions of gas molecules
Random collisions of gas molecules
Kinetic theory of gas:
Kinetic theory of gas states that the gas is made up of very large number of particles that are in constant, random motion.
These particles collide with each other and with the walls of the container. This theory is used to describe the macroscopic
properties (pressure, temperature, volume etc.) of the ideal gas.
Applications of gases:
- Gases like oxygen, nitrous oxides, liquid nitrogen are frequently used in health care industry. Nitrous oxide is used as general anesthesia, while liquid nitrogen is used for the cryogenic preservation of organs, tissues and blood products.
- Carbon dioxide is responsible for the fizziness in the soft drinks.
- Chloro fluoro carbon (CFC) or tetrafluoroethane (used in modern refrigerators) is used as coolant in refrigerators.
- Aerosols (solid or liquid particles suspended in the gas) are used in the treatment of respiratory disorders.