 Geometry Of Methane
Geometry Of Methane
Valence electrons determine molecular shape. Molecules are three–dimensional entities and therefore best depicted in three dimensions.
We can translate the two–dimensional electron–dot structure representing a molecule into a more accurate three–dimensional rendering by using the model known as valence shell electron–pair repulsion, also called VSEPR (pronounced ves–per).
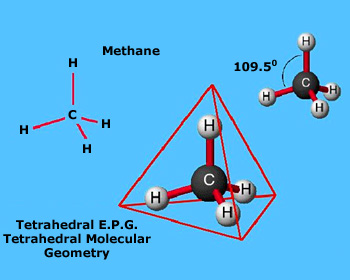
According to this model, any given pair of valence–shell electrons strives to get as far away as possible from all other electron pairs in the shell. This includes both non–bonding pairs and any bonding pairs not taking part in a double or triple bond. (Pairs in a multiple bond stay together because of their mutual attractions for the same two nuclei). The VSEPR model talks about the repulsion between pairs of electrons, not between the two electrons in a pair (the electrons in a pair don't repel each other because of their opposite spins). It is this striving for maximum separation distance between electron pairs that determines the geometry of any molecule. The two dimensional electron–dot structure for methane, CH4, is shown in adjacent figure.
 Geometry and molecular shapes of the molecules
Geometry and molecular shapes of the molecules
In this structure, the bonding electron pairs (shown as straight lines representing one electron from each atom) are set 90° apart because that is the farthest apart they can be shown in two dimensions. When we extend to three dimensions, however, we can create a more accurate rendering in which the four bonding pairs are 109.5° apart.
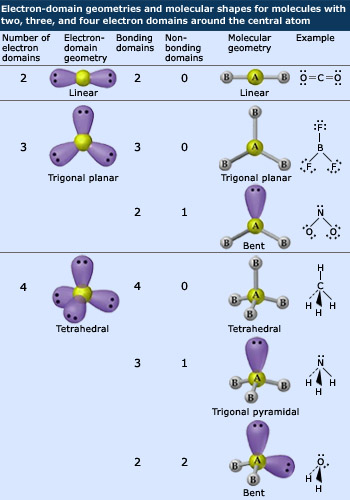
The VSEPR model allows us to use electron–dot structures to predict the three–dimensional geometry of simple molecules. This geometry is determined by considering the number of substituents surrounding the central atom. A substituent is any atom or non–bonding pair of electrons. The carbon of the methane molecule, for example, has four substituents – the four hydrogen atoms. The oxygen atom of a water molecule also has four substituents – two hydrogen atoms and two non–bonding pairs of electrons.
Note that each non–bonding pair is shown inside a lobe–shaped atomic orbital. An atomic orbital is the volume of space in which a pair of electrons may be found most of the time. These lobes are shown here to illustrate the role that non–bonding electron pairs play in determining the geometry of a molecule.
When a central atom has only two substituents, the geometry of the molecule is linear, meaning a single straight line may be drawn passing through both substituents and the central atom. Three substituents arrange themselves in a triangle the plane of which passes through the central atom and so this molecular geometry is called triangular planar. Four substituents form a tetrahedron, as already discussed. Five substituents result in a triangular bipyramidal geometry, which is two triangle based pyramids sharing a base and having the two apexes pointing in opposite directions. Six substituents arrange themselves around the central atom in a geometry that, if it had a surface, would show eight sides. To indicate this eight–sided geometry, this structure is called octahedral. All these are the geometries that allow for maximum distance between substituents.