 Availability of empty d orbitals make the electrons of s and p orbitals to excite to the empty orbitals and
result in the hybridization of orbitals resulting in octahedral geometry in the molecules.
Availability of empty d orbitals make the electrons of s and p orbitals to excite to the empty orbitals and
result in the hybridization of orbitals resulting in octahedral geometry in the molecules.
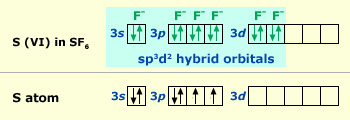
Intermixing of one ‘s’, three ‘p’ and two ‘d’ orbitals of almost same energy by giving six identical and degenerate hybrid orbitals is called sp3d2 hybridization.
These six sp3d2 orbitals are arranged in octahedral symmetry by making 90° angles to each other. This arrangement can be visualized as four orbitals arranged in a square plane and the remaining two are oriented above and below this plane perpendicularly.
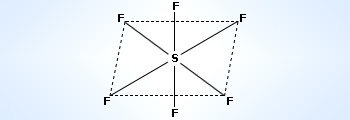 Geometry of SF6 molecule
Geometry of SF6 molecule
Example: SF6
In SF6, one electron each from 3s and 3p orbitals is promoted to 3d orbitals The six orbitals get hybridized to form six sp3d2 hybrid orbitals. Each of these sp3d2 hybrid orbitals overlaps with 2p orbital of fluorine to form S–F bond.
Thus, SF6 molecule has octahedral structure. The dotted electrons represent electrons from F–atoms.