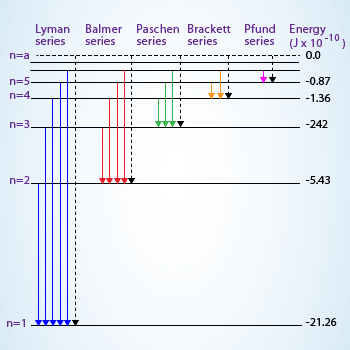
 Transition of electron from higher energy level to the first level will result in Lyman series in which
the lines correspond to the ultra violet region of electromagnetic spectrum, to the second level result in visible region
(Balmer series), infrared region to the third and successive levels there on.
Transition of electron from higher energy level to the first level will result in Lyman series in which
the lines correspond to the ultra violet region of electromagnetic spectrum, to the second level result in visible region
(Balmer series), infrared region to the third and successive levels there on.
When atoms are heated or subjected to an electric discharge, they absorb energy, which is subsequently emitted as radiation. For example, if sodium chloride is heated in the flame of Bunsen burner, sodium atoms are produced which gives out a characteristic yellow flame coloration (there are two lines in emission spectrum of sodium corresponding to wavelengths of 589 nm and 589.6 nm). Spectroscopy deals with the study of either radiation absorbed or radiation emitted. Atomic spectroscopy is an important technique for studying the energy and the arrangement of particles in the atoms.
Hydrogen atomic spectra:
The important result of the quantum explanation of the atomic structure was that it was able to explain atomic spectra
of elements.
The easiest atomic spectrum to understand is the hydrogen atomic spectra. The hydrogen atoms are heated in a tube. The heat excites the electrons to higher orbitals. When the electrons return to lower orbitals, they emit photons. The line emission of hydrogen spectra have a series–each series is known as Lyman, Balmer, Paschen, Brackett, Pfund, etc., depending on the final shell where the electron drop to.
A single atom can emit only one photon during one transition of its electron. In fact, a very large number of atoms are involved in the production of the spectrum. At any instant, there are many atoms undergoing the same transition and different groups of atoms undergoing different transitions. The combined effect of all the transitions is the continuous production of various lines of the spectrum.