 Cathode Ray Experiment
Cathode Ray Experiment
After Dalton's breakthrough of atomic theory, scientists tried to determine the masses of atoms from the fraction of elements in compounds. Since an individual atom is so small, the mass of the atoms of one of the element was determined relative to the mass of the atoms of another element, based on a mass standard. Dalton's model is very important because it was based on the observation of many chemical reactions and the masses of reacting elements which could be explained in terms of atoms. But for the explanation of drawbacks that are discussed need a more complex atomic model. The discovery of fundamental (subatomic) particles leads to the nuclear model of an atom.
 Thomson's Cathode Ray Tube
Thomson's Cathode Ray Tube
Discovery of Electrons
Cathode rays are a type of radiation emitted by the negative terminal, the cathode, and was discovered by passing electricity
through glass tubes from which the air was mostly evacuated. One device used to investigate this phenomenon was a cathode ray
tube, the forerunner of the television tube. It is a glass tube from which most of the air has been evacuated. When the two metal
plates are connected to a high–voltage source, the negatively charged plate, called the cathode, emits an invisible ray.
The cathode ray is drawn to the positively charged plate, called the anode, where it passes through a hole and continues
traveling to the other end of the tube. When the ray strikes the specially coated surface, it produces a strong fluorescence, or
bright light.
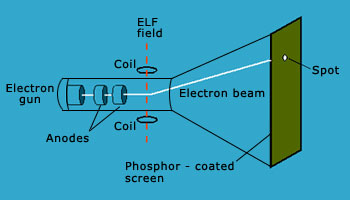
In some experiments, two electrically charged plates and a magnet were added to the outside of the cathode ray tube. In the presence of magnetic field, the cathode ray strikes point ‘B’ whereas in the presence of electric field, the cathode ray strikes point ‘A’ as shown in the figure. In the presence or absence of both electric and magnetic fields such that their magnitudes cancel out each other, the cathode ray strikes point 'C'. According to electromagnetic theory, a moving charged body behaves like a magnet and can interact with electric and magnetic field through which it passes. Because the cathode ray is attracted by the plate bearing positive charges and repelled by the plate bearing negative charges, it must consist of negatively charged particles. These negatively charged particles are called as electrons.
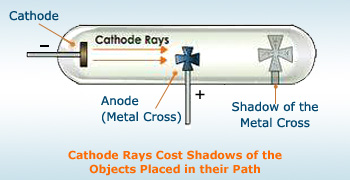
- Cathode rays travel in straight lines. That is why, cathode rays cast shadow of any solid object placed in their path. The path cathode rays travel is not affected by the position of the anode.
- Cathode rays consist of matter particles, and posses energy by the virtue of its mass and velocity. Cathode rays set a paddle wheel into motion when it is placed in the path of these rays one the bladder of the paddle wheel.
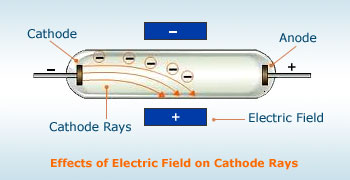
Cathode rays consist of negatively charged particles. When cathode rays are subjected to an electrical field, these get deflected towards the positively charge plate(Anode).
We know that a positively charged body would attract only a negatively charged body, therefore the particles of cathode rays carry negative charge.
Cathode rays also get deflected when these are subjected to a strong magnetic field.
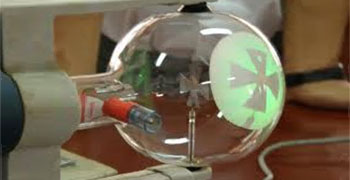 Glass Surface Showing Green Fluorescence
Glass Surface Showing Green Fluorescence
- Cathode rays heat the object only which they fall. The cathode ray particles possess kinetic energy. When these particles strike an object, a part of the kinetic energy is transferred to the object this causes a rise in the temperature of the object.
- Cathode rays cause green fluorescence on glass surface, i.e., the glass surface only which the cathode rays strike show a colored shine.
- Cathode rays can penetrate through thin metallic sheets.
- Cathode rays ionize the gases through which they travel.
- Cathode rays travel with speed nearly equal to that of light.