 Niels Bohr
A Danish physicist made fundamental contributions to understand the structure of an atom and quantum
mechanics. He was awarded Nobel prize in 1922 for his pioneering contribution to atomic structure of atom.
Niels Bohr
A Danish physicist made fundamental contributions to understand the structure of an atom and quantum
mechanics. He was awarded Nobel prize in 1922 for his pioneering contribution to atomic structure of atom.
Bohr's Atomic Model :
Niels Bohr was Rutherford's student. He tried to combine
Rutherford's results with the already known facts about atoms. The nature of light was extensively investigated. In 1900,
a German physicist Max Planck hypothesized that light energy was quantized, and it came in chunks called photons.
 Max Planck proposed that light travels in the form of quanta. Einstein has given explanatory proof for
theory proposed by Planck. Both were instrumental in explaining the nature of light.
Max Planck proposed that light travels in the form of quanta. Einstein has given explanatory proof for
theory proposed by Planck. Both were instrumental in explaining the nature of light.
Planck recognized that a beam of light energy is not the continuous (non quantized) stream of energy as we think of it. Instead the beam consists of zillions of small, discrete packets of energy, each packet is called as a quantum, as represented in the figure. This was later confirmed when Einstein was able to explain the photoelectric effect. Thus the concept of light photon came to be accepted.
Light is quantized, which means it consists of a stream of energy packets. Each packet is called a quantum, also known as a photon. Bohr took these results together. If an atom is emitting light (which comes in packets or photons) when it is heated, then the light (the photons) must be coming because the electrons are changing their energies, which again have to be in discrete packets! This was a major breakthrough in understanding the structure of the atom.
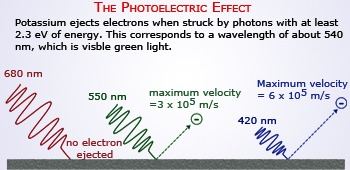 Photoelectric effect which explained the particle nature of light. When the metals hit with photons of
definite energy results in the ejection of electrons.
Photoelectric effect which explained the particle nature of light. When the metals hit with photons of
definite energy results in the ejection of electrons.
To explain his idea of atomic spectra, based on the structure of atoms, Bohr made a few postulates. This was due to his following arguments: Bohr recognized that the potential energy of an electron in an atom depends on the electron's distance from the nucleus. Secondly, Bohr recognized that when an atom absorbs a photon of light, it is absorbing energy. This energy is acquired by one of the electrons surrounding the atom's nucleus. Because this electron has gained energy, it must move away from the nucleus.
A photon of light is absorbed by the electron and the electron gains potential energy and moves farther from nucleus. A photon of light is emitted by the electron and the electron loses potential energy and moves closer to nucleus. Bohr reasoned that because light energy is quantized, the energy of an electron in an atom must also be quantized. When you are on a staircase, you are restricted to where the steps are you cannot stand at a height that is, say, halfway between any two adjacent steps. Similarly, there are only a limited number of permitted energy levels in an atom, and an electron can never have an amount of energy between these permitted energy levels.
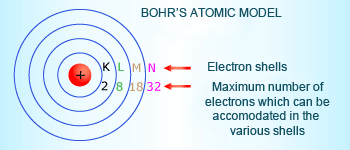 Bohr's Atomic Model
Bohr's Atomic Model
Bohr's Postulates:
Two of the Bohr's postulates are as follows:
- An electron can revolve only in fixed orbits around the nucleus.
- An orbiting electron does not radiate energy, even though it is in an accelerated motion.
Bohr gave each electron energy level a principal quantum number n, where n is always some integer. The lowest energy level has a principal quantum number n= 1. An electron for which n =1 is as close to the nucleus as possible, and an electron for which n= 2, n= 3, and so forth is farther away from the nucleus. Bohr saw this as similar to how the planets are held in orbit around the sun at given distances from the sun.Bohr's quantized model of the atom thus became known as the planetary model.
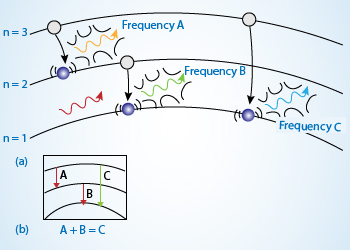 Electron when jump from higher energy orbits to lower energy orbits emit the excess energy in the form of
radiation which is given as spectral line in emission spectra.
Electron when jump from higher energy orbits to lower energy orbits emit the excess energy in the form of
radiation which is given as spectral line in emission spectra.
The frequency of light emitted (or absorbed) by an atom is proportional to the energy difference between electron orbits. Because the energy differences between orbits are discrete, the frequencies of light emitted (or absorbed) are also discrete. The electron here can emit only three discrete frequencies of light – A, B, and C. The greater the transition, the higher the frequency of the photon emitted.
The sum of the energies (and frequencies) for transitions A and B equals the energy (and frequency) of transition C. By utilizing Planck's quantum hypothesis, Bohr's model solved the mystery of atomic spectra, etc. But many questions still remained unanswered. For example, this model was unable to explain the fine structures in the atomic spectra or why different energy levels in an atom had different number of electrons. This was answered when a new mechanics for subatomic particle, called quantum mechanics, was applied.