Mole Fraction(X)
States of Matter > Solutions
 Mole Fraction(X)
Mole fraction of sodium hydroxide in sodium hydroxide solution, is the ratio of the number of moles of
the sodium hydroxide to the total number of moles of components in the solution.
Mole Fraction(X)
Mole fraction of sodium hydroxide in sodium hydroxide solution, is the ratio of the number of moles of
the sodium hydroxide to the total number of moles of components in the solution.
The mole fraction of a solute is the ratio of number of moles of solute to the total number of moles (solute and solvent), that is parts by mole.
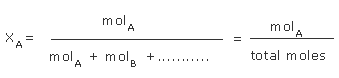
A solution with mole fraction units is prepared by measuring the desired number of moles of each component and then mixing.
Mole fraction is used very frequently in the construction of phase diagrams. It has a number of advantages:
- It is not temperature dependent (such as molar concentration) and does not require knowledge of the densities of the phase(s) involved.
- A mixture of known mole fraction can be prepared by weighing off the appropriate masses of the constituents.
- In a mixture of ideal gases, the mole fraction can be expressed as the ratio of partial pressure to total pressure of the mixture.