Molality(m)
States of Matter > Solutions
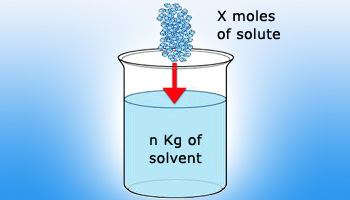 Image Showing X moles of solute added to n kg of solvent
Image Showing X moles of solute added to n kg of solvent
A concentration term that does not contain volume in its ratio is molality. Molality is defined as the number of moles of solute dissolved in 1 kilogram of solvent and is abbreviated as the lower case letter ‘m’.

Molality includes the quantity of solvent, but not the solution. Molal solutions are prepared by measuring masses of solute and solvent. Mass do not change with temperature, so as molality.