 A crystal lattice may be imagined to be made up of a very large number of small units known as unit cells.
A unit cell is a smallest repeating unit in a crystal lattice which when repeated again and again in different directions results
in the crystal of the given substance. Shapes of crystals depends upon the length of intersecting edges i.e., a, b, c and also
the angles (α, β and γ) between the edges.
A crystal lattice may be imagined to be made up of a very large number of small units known as unit cells.
A unit cell is a smallest repeating unit in a crystal lattice which when repeated again and again in different directions results
in the crystal of the given substance. Shapes of crystals depends upon the length of intersecting edges i.e., a, b, c and also
the angles (α, β and γ) between the edges.
Solids are the thickest forms of matter. All the molecules in a solid are tightly fitted together, so the molecules can't move around very much. Based on the molecular or atomic arrangement solids are broadly classified into crystalline solids and amorphous solids.
Crystalline solid structures may be defined based on the attractive forces that hold them together or on the arrangement of the atoms in the crystals themselves. Crystal types are based on attractive forces.
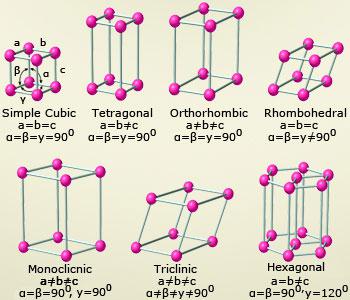
Crystals grouped by lattices (shapes):
A lattice is a regular arrangement of particles whether
these are atoms, ions or molecules. Seven types of basic
crystal structures are present. They are.
Cubic or isometric:
These are the crystals lattices with three equal axes,
intersecting at right angles to each other.
- Hexagonal: These are the crystal lattices with three equal axes, intersecting at 60° angle in a horizontal lane and a fourth, longer or shorter axis, perpendicular to the plane of the other three.
- Tetragonal: These are similar to cubic crystals with two equal, horizontal axes at right angles and one axis longer or shorter than the other two axis and is perpendicular to the plane. They form the pyramids and prisms with this type of arrangement.
- Orthorhombic: In this type of crystals, the three axes are unequal and they intersect at right angles to each other. They form rhombic prisms or di–pyramids.
- Monoclinic: In this arrangement, the three axes are unequal and two axes intersect at right angles while the third is inclined at an oblique angles to the plane of other two. They look like the skewed tetragonal crystals.
- Trigonal or Rhombohedral: In this arrangement, the three axis are equal in length and the three axis will intersect at oblique angles.
- Triclinic: In this type, the three axis are not equal and they intersect at oblique angles with each other.
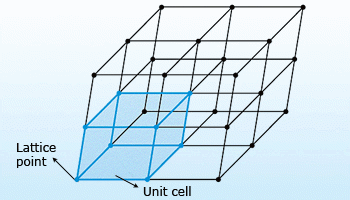 Unit cell: The simplest repeating unit in a crystal is called a unit cell. The unit cells
stacked in three–dimensional space describe the bulk arrangement of atoms of the crystal. The crystal structure has a
three–dimensional shape.
Lattice points: Points in a space which are occupied by the constituents of that unit cell (atoms or ions) are
called lattice points. These points form a regular pattern throughout the crystal that is called crystal lattice.
Unit cell: The simplest repeating unit in a crystal is called a unit cell. The unit cells
stacked in three–dimensional space describe the bulk arrangement of atoms of the crystal. The crystal structure has a
three–dimensional shape.
Lattice points: Points in a space which are occupied by the constituents of that unit cell (atoms or ions) are
called lattice points. These points form a regular pattern throughout the crystal that is called crystal lattice.
Crystals grouped by bonding
(properties):
These class of crystals are grouped by taking the
properties exhibited by them due to their bonding.
These are of following types:
- Metallic crystals: A crystalline solid in which the atoms are held together by metallic bonds. Metallic crystals are found in some interstitial compounds as well as in metals and alloys.
- Ionic Crystal: A crystal in which the lattice–site occupants are charged ions held together primarily by their electrostatic interaction.
- Molecular Crystal: A solid consisting of a lattice array of molecules such as hydrogen, methane, or more complex organic compounds, bound by weak van der Waals forces, and therefore retaining much of their individuality.
- Covalent Crystal: A crystal held together by covalent bonds. Also known as valence crystal.
A particular solid (element or compound) has a specific arrangement of atoms, which is called as its crystal structure. Studies of crystalline solids show that they consist of identical units arranged in a periodic manner. Since a crystal consists of identical units arranged in a periodic array, it is not necessary to give the positions of all its atoms to describe the structure of a crystal.
Unit cell:
The smallest building block of a crystal, consisting of atoms,
ions, or molecules, whose geometric arrangement defines a
crystal's characteristic symmetry and whose repetition
in space produces a crystal lattice.
Crystal Lattice:
A geometric arrangement of the points in space at which
the atoms, molecules, or ions of a crystal occur. Also called space lattice.
Bravais lattices:
A 3–dimensional geometric arrangement of the atoms or molecules
or ions composing a crystal
14 Bravais lattices are found in nature, thus 14 types of arrangements
are possible for crystals.
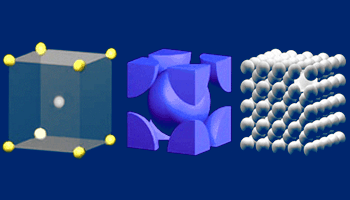 The BCC unit cell
The BCC unit cell consists of a net total of two atoms; one in the center and eight eighths from corners
as shown in the middle. The image highlights a unit cell in a larger section of the lattice.
The BCC unit cell
The BCC unit cell consists of a net total of two atoms; one in the center and eight eighths from corners
as shown in the middle. The image highlights a unit cell in a larger section of the lattice.
Primary metallic crystalline structures
(BCC, FCC, HCP):
There are 14 different types of crystal unit cell structures or
lattices are found in nature. However, most metals and many other
solids have unit cell structures described as body center cubic (BCC),
face centered cubic (FCC) or Hexagonal Close Packed (HCP).
Body–Centered Cubic (BCC) structure:
The body–centered cubic unit cell has atoms at each of the eight
corners of a cube and one atom in the center of the cube. Each of the
corner atoms is the corner of another cube so the corner atoms are shared
among eight unit cells. It is said to have a coordination number of 8.
Metals which have a BCC structure are usually harder and less malleable than
close–packed metals such as gold.
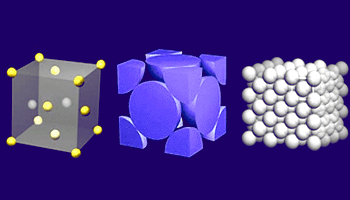 Close cross sectional view of FCC
The FCC unit cell consists of a net total of four atoms; eight eighths from corners and six halves of
the face atoms make total of 4 atoms.
Close cross sectional view of FCC
The FCC unit cell consists of a net total of four atoms; eight eighths from corners and six halves of
the face atoms make total of 4 atoms.
Face Centered Cubic (FCC) structure:
The face centered cubic structure has atoms located at each of the corners
and the centers of all the cubic faces. Each of the corner atoms is the corner
of another cube so the corner atoms are shared among eight unit cells.
Additionally, each of its six face centered atoms is shared with
an adjacent atom. Since 12 of its atoms are shared, it is said to
have a coordination number of 12.
Some of the metals that have the FCC structure include aluminum, copper,
gold, iridium, lead, nickel, platinum and silver.
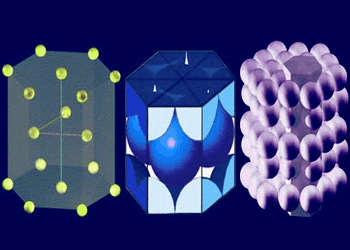 Close cross sectional view of HCP
There are six atoms in the HCP unit cell. Each of the 12 atoms in the corners of the top and bottom
layers contribute 1/6 atom to the unit cell, the two atoms in the center of the hexagon of both the top and bottom layers each
contribute 1/2 atom and each of the three atom in the middle layer contribute 1 atom.
Close cross sectional view of HCP
There are six atoms in the HCP unit cell. Each of the 12 atoms in the corners of the top and bottom
layers contribute 1/6 atom to the unit cell, the two atoms in the center of the hexagon of both the top and bottom layers each
contribute 1/2 atom and each of the three atom in the middle layer contribute 1 atom.
Hexagonal Close Packed (HCP) structure:
The hexagonal structure of alternating layers is shifted so its
atoms are aligned to the gaps of the preceding layer. The atoms
from one layer nest themselves in the empty space between the atoms
of the adjacent layer just like in the FCC structure. However, instead
of being a cubic structure, the pattern is hexagonal.
The coordination number of the atoms in this structure is 12. There are six nearest neighbors in the same close packed layer, three in the layer above and three in the layer below. The HCP structure is very common for elemental metals and some examples include beryllium, cadmium, magnesium, titanium, zinc and zirconium.
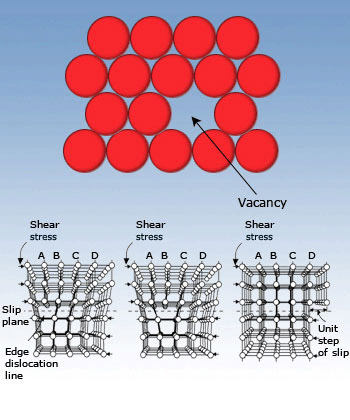 Point defects are due to the absence of atom in a regular lattice structure. Point defects are common in
crystals with large anions such as AgBr, AgI, RbAgI4. Due to the defects, the ions have some freedom to move about in
crystals, making them relatively good conductors.
Point defects are due to the absence of atom in a regular lattice structure. Point defects are common in
crystals with large anions such as AgBr, AgI, RbAgI4. Due to the defects, the ions have some freedom to move about in
crystals, making them relatively good conductors.Edge dislocation is responsible for the ductility and malleability. In fact the hammering and stretching of materials often involve the movement of edge dislocation shown in the figure.
Anisotropy:
In a single crystal, the physical and mechanical properties often differ
with orientation. It can be seen from looking at our models of crystalline
structure that atoms should be able to slip over one another or distort
in relation to one another easier in some directions than others. When
the properties of a material vary with different crystallographic
orientations, the material is said to be anisotropic.
Crystal Defects:
Crystal defect is imperfection in the regular geometrical
arrangement of the atoms in a crystalline solid.
There are basic classes of crystal defects:
- Point defects, which are places where an atom is missing or irregularly placed in the lattice structure. Point defects include lattice vacancies, self–interstitial atoms, substitution impurity atoms, and interstitial impurity atoms.
- Linear defects, which are groups of atoms in irregular positions. Linear defects are commonly called dislocations.
- Planar defects, which are interfaces between homogeneous regions of the material. Planar defects include grain boundaries, stacking faults and external surfaces.
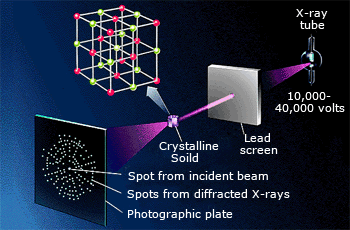 In X–ray crystallography, an X–ray beam is diffracted by a crystal. The diffraction pattern
can be recorded as spots where the diffracted X–rays strike a photographic plate.
In X–ray crystallography, an X–ray beam is diffracted by a crystal. The diffraction pattern
can be recorded as spots where the diffracted X–rays strike a photographic plate.
X–Ray Diffraction:
A Tool to Study Crystal Structures:
When atoms in a crystal are hit with X–rays, they absorb some
of the radiation and then emit it again in all directions.
In this effect, each atom becomes a tiny X–ray source. The
X–rays emitted by such atoms will have two types of scattering,
one type of rays which are in–phase with each other and the
other type which are out of phase. The constructive (in–phase)
and destructive (out–of–phase) interferences create a phenomenon
called diffraction. X–Ray diffraction by crystals has enabled many
scientists to win Nobel prizes by determining the structures of
extremely complex compounds in a particularly elegant way.
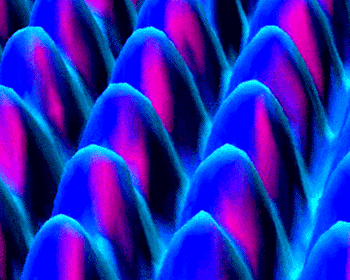 Alignment of atoms on platinum surface as shown by STM at IBM research center.
Alignment of atoms on platinum surface as shown by STM at IBM research center.
In a crystal, there is enormous number of atoms, evenly spaced throughout the lattice. When the crystal is bathed in X–rays, intense beams are diffracted because of constructive interference, and they appear only in specific directions. In other directions, no X–rays appear because of destructive interference. When the X–rays coming from the crystal are directed onto the photographic film, the diffracted beams form a diffraction pattern which is recorded on the photographic film. The film is darkens only where the X–rays strike.