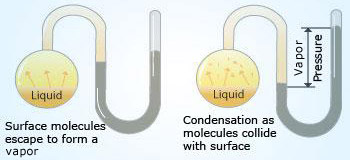
 Liquid–gas equilibrium
In a closed flask at constant temperature with the air removed, the initial pressure is zero. As molecules leave the surface and enter the space above the liquid, the pressure rises.
Liquid–gas equilibrium
In a closed flask at constant temperature with the air removed, the initial pressure is zero. As molecules leave the surface and enter the space above the liquid, the pressure rises.At equilibrium, the same number of molecules leaves as enter the liquid within a given time, so the pressure of the vapor reaches a constant value.
What happens at the molecular level during evaporation? In the beginning, the traffic is only one way: Molecules are moving from the liquid to the empty space. Soon the molecules in the space above the liquid establish a vapor phase.
The rate of evaporation is constant at any given temperature and the rate of condensation increases with the increasing concentration of molecules in the vapor phase. A state of dynamic equilibrium, in which the rate of a forward process is exactly balanced by the rate of the reverse process, is reached when the rates of condensation and evaporation become equal. The equilibrium vapor pressure is the vapor pressure measured when a dynamic equilibrium exists between condensation and evaporation.
It is important to note that the equilibrium vapor pressure is the maximum vapor pressure of a liquid at a given temperature and that it is constant at a constant temperature. (It is independent of the amount of liquid as long as there is some liquid present).
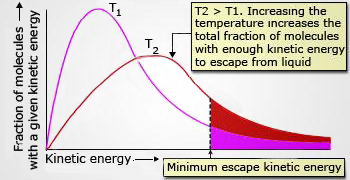 As the temperature increases, the most probable velocities increase. The fraction with enough energy to escape is greater at high temperature showing that evaporation increases with increase in temperature.
As the temperature increases, the most probable velocities increase. The fraction with enough energy to escape is greater at high temperature showing that evaporation increases with increase in temperature.
Factors determining vapor pressure:
Two factors helps in governing the vapor pressure of a liquid.
- Temperature
- Chemical composition
- Temperature:
Once a particular liquid is selected then only the temperature has to be considered as the vapor pressure of a given liquid is a function solely of its rate of evaporation “per unit area of liquid's surface”. When the rate is large, a large concentration of molecules in the vapor state is necessary to establish equilibrium. As the temperature of a given liquid increases, so does its rate of evaporation and it will eventually lead to the increase in vapor pressure.
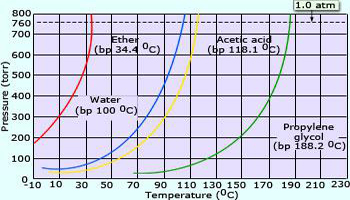 Propylene glycol has the highest vapor pressure among the other liquids shown in graph, because of the reason that its intermolecular forces are strongest followed by acetic acid then by water and ether respectively. Hence, propylene glycol is difficult to vaporize.
Propylene glycol has the highest vapor pressure among the other liquids shown in graph, because of the reason that its intermolecular forces are strongest followed by acetic acid then by water and ether respectively. Hence, propylene glycol is difficult to vaporize.
- Chemical composition:
Chemical composition changes from one liquid to another. The strengths of intermolecular attractions differ from one to another. In the given adjacent figure, we find that intermolecular attractions are strongest in propylene glycol and next strongest in acetic acid, third strongest in water and the weakest in ether.
Vapor pressure neither depend on the total surface area of the liquid, nor on the volume of the liquid in the container, nor on the volume of container itself, just as long as some liquid remain when equilibrium is reached. The reason for these fractions not influencing is that none of the factors affects the rate of evaporation per unit surface area.