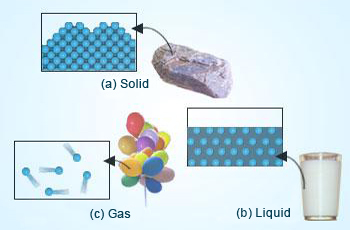
There are actually five states of matter – solid, liquid, gas (usually these three states exist at room temperature); the fourth state of matter is plasma (which forms at very high temperatures, when electrons are stripped off from the atoms. For example, the matter in the Sun is in plasma state) and the fifth state of matter occurs at very low temperatures. It is called as the Bose–Einstein condensate.
Matter in a gaseous state is neither in a definite shape nor volume. It is very ephemeral. If it is kept in a container, it fills the container completely! The container volume in which it is placed defines the gaseous state of a substance.
Changes in states of matter – Effect of temperature:
- Conversion of liquid to vapor: When a liquid is heated, the heat energy makes the liquid particles move even faster. At the boiling point the particles of a liquid has sufficient kinetic energy to overcome the forces of attraction holding them together and separates into individual particles and the liquid boils to form a gas.
- Gas to liquid conversion (Condensation): If we cool steam (or water vapor) by lowering its temperature, it is converted into liquid water. The process of converting a gas to a liquid, by cooling is called condensation. Condensation is reverse of boiling (or vaporization). The heat energy which has to be supplied to change the state of a substance is called its latent heat.
- Latent heat of vaporization (Liquid to gas conversion): The heat required to convert a liquid into the vapor state (or gas) is called latent heat of vaporization.
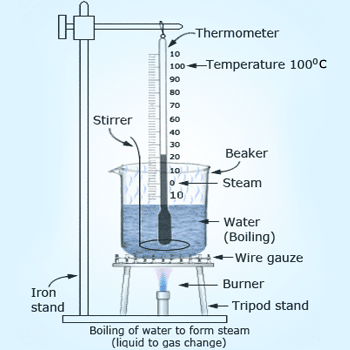 Latent heat of vaporization experiment.
At 100°C water absorbs heat but there will be
no rise in temperature is observed showing that the heat supplied is
utilized in converting water into vapor.
Latent heat of vaporization experiment.
At 100°C water absorbs heat but there will be
no rise in temperature is observed showing that the heat supplied is
utilized in converting water into vapor.
Experiment to understand the phenomenon of
latent heat of vaporization:
Take some water in a beaker and suspend a thermometer in it.
Heat the water by using a burner and note its temperature after
every minute. As heat is given, the temperature of water rises
gradually until 100°C is reached. At 100°C, water boils and starts
changing into steam (which is a gas). As more heat is given to water,
more steam is formed and the thermometer reading remains constant
at 100°C, it shows that there is no rise in temperature during
the boiling of water. Thus, once the water has begun to boil, the
temperature remains constant at 100°C until all the water has
changed into steam. The heat which is going into boiling water
but not increasing its temperature is the energy required to
change the state of water from liquid to vapor is known as
latent heat of vaporization of water.
- The latent heat of vaporization of a liquid is the quantity of heat in joules required to convert 1 kilogram of liquid (at its boiling point) to vapor or gas, without any change in temperature.
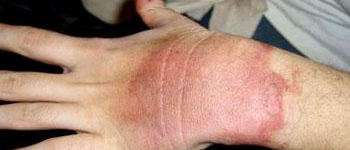 These burns have been caused by steam.
Burns caused by steam are much more severe than
water burns at same temperature due to the fact that steam has more amount
of heat called latent heat of vaporization stored in it when compared with
water at 100°C.
These burns have been caused by steam.
Burns caused by steam are much more severe than
water burns at same temperature due to the fact that steam has more amount
of heat called latent heat of vaporization stored in it when compared with
water at 100°C.
Examples in our daily life of latent heat of vaporization:
- If a little volume of spirit is applied at the back side of our hand, and wave it around, the spirit evaporates rapidly and our hand feels very cold. It is due to the fact that spirit needs latent heat of vaporization to change from liquid to vapor state. The spirit takes the latent heat of vaporization from our hand. So, our hand loses heat and gets cooled.
- Burns caused by steam are much more severe than those caused by boiling water though both of them are at the same temperature of 100°C. Its due to the fact that steam contains more heat, in the form of latent heat, than boiling water. So, when steam falls on our skin and condenses to produce water it gives out 22.5 × 105 joules per kilogram more heat than boiling water at the same temperature. Since steam gives out more heat than boiling water, it causes more severe burns.