 Image showing symmetrical distribution of electrons and unsymmetrical distribution of electrons.
Image showing symmetrical distribution of electrons and unsymmetrical distribution of electrons.
The London dispersion force is the weakest intermolecular force. The London dispersion force is a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles. This force is sometimes called an induced dipole–induced dipole attraction. London forces are the attractive forces that cause nonpolar substances to condense to liquids and to freeze into solids when the temperature is lowered sufficiently. Because of the constant motion of the electrons, an atom or molecule can develop a temporary (instantaneous) dipole when its electrons are distributed unsymmetrically about the nucleus.
Dispersion forces are present between any two molecules (even polar molecules) when they are almost touching.
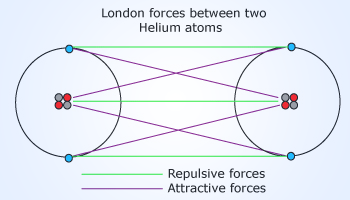 London forces in ‘He’ molecule
Motion of electrons in between the closely
spaced atoms of He results in polarization of charge. This results
in formation of dipoles between the He atoms resulting in the forces
of attraction called as London force. This is responsible for the
non–polar substances to become liquids under low temperatures.
London forces in ‘He’ molecule
Motion of electrons in between the closely
spaced atoms of He results in polarization of charge. This results
in formation of dipoles between the He atoms resulting in the forces
of attraction called as London force. This is responsible for the
non–polar substances to become liquids under low temperatures.
Molecular Size:
Dispersion forces are present between all molecules, whether
they are polar or nonpolar.
- Larger and heavier atoms and molecules exhibit stronger dispersion forces than smaller and lighter ones.
- In a larger atom or molecule, the valence electrons are, on average, farther from the nuclei than in a smaller atom or molecule. They are less tightly held and can more easily form temporary dipoles.
- The ease with which the electron distribution around an atom or molecule can be distorted is called the polarizability.
 van der Waal force
Gecko can adhere to almost everything except
Teflon [polytetrafluoroethylene] due to van der Waal force between
its feet and surface to which it is adhered.
van der Waal force
Gecko can adhere to almost everything except
Teflon [polytetrafluoroethylene] due to van der Waal force between
its feet and surface to which it is adhered.
London dispersion forces tend to be:
- Stronger between molecules that are easily polarized.
- Weaker between molecules that are not easily polarized.
Molecular Shape:
The shapes of molecules also affect the magnitudes of dispersion
forces between them.
- At room temperature, neopentane (C5H12) is a gas whereas n–pentane (C5H12) is a liquid.
- London dispersion forces between n–pentane molecules are stronger than those between neopentane molecules even though both molecules are nonpolar and have the same molecular weight.
- The somewhat cylindrical shape of n–pentane molecules allows them to come in contact with each other more effectively than the somewhat spherical neopentane molecules.