Life blood of Biosphere
States of Matter > Air, Water & Soil
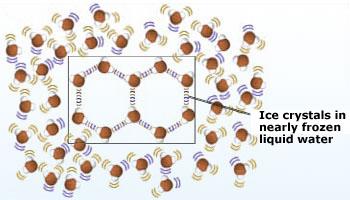 Crystals of ice is seen in water at 0°C. In ice, each water molecule is hydrogen – bonded to four
neighboring water molecules in a three dimensional crystal. Because the crystal is spacious, ice has fewer molecules than an
equal volume of liquid water. In other words, ice is less dense than liquid water. The open structure of these crystals makes
the volume of the water slightly greater than it would be without the crystals
Crystals of ice is seen in water at 0°C. In ice, each water molecule is hydrogen – bonded to four
neighboring water molecules in a three dimensional crystal. Because the crystal is spacious, ice has fewer molecules than an
equal volume of liquid water. In other words, ice is less dense than liquid water. The open structure of these crystals makes
the volume of the water slightly greater than it would be without the crystals
Water's properties contribute to the suitability of Earth as an environment for life. These are water's cohesive behavior, its ability to moderate temperature, its expansion upon freezing, and its versatility as a solvent.
Water is a remarkable substance with a unique combination of properties:
- There are strong forces of attraction (called hydrogen bonds) between molecules of water. These attractive forces are the major factor determining water's unique properties. Water exists as a liquid over a wide temperature range because of the strong forces of attraction between water molecules. Its high boiling point of 100°C (212°F) and low freezing point of 0°C (32°F) mean that water remains as a liquid in most climates on the Earth. The strong attractive forces between the molecules of liquid water cause its surface to contract (high surface tension) and adhere to and coat a solid (high wetting ability). These cohesive forces pull water molecules at the surface layer together so strongly that it can support small insects. The combination of high surface tension and wetting ability allow water to rise through a plant from the roots to the leaves (capillary action).
 The massive bear is supported by the floating ice.
The massive bear is supported by the floating ice.
- Liquid water changes temperature very slowly because it can store a large amount of heat without a large change in temperature. This high heat capacity helps protect living organisms from temperature fluctuations, moderates the Earth's climate and makes water an excellent coolant for car engines, power plants and heat – producing industrial processes. It takes a lot of heat to evaporate liquid water because of the strong forces of attraction between its molecules. Water absorbs large amounts of heat as it changes into water vapor and releases this heat as the vapor condenses back to liquid water. This is a primary factor in distributing heat throughout the world and thus plays an important role in determining the climates of various areas. This property also makes water evaporation an effective cooling process, which is why we feel cooler when perspiration or bathwater evaporates from our skin.
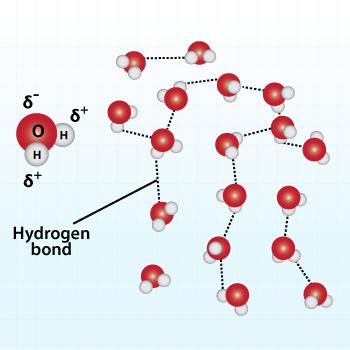 Hydrogen bonding between water molecules
Hydrogen bonding between water molecules
- Liquid water can dissolve a variety of compounds. This enables it to carry dissolved nutrients into the tissues of living organisms, flush waste products out of those tissues, serve as an all – purpose cleanser and helps to remove and dilute the water – soluble wastes. Water's superiority as a solvent also means that water – soluble wastes pollute it easily.
- Water molecules can break down (ionize) into hydrogen ions (H+) and hydroxide ions (OH− ), which helps to maintain a balance between acids and bases in cells, as measured by the pH of water solutions.
- Unlike most liquids, water expands when it freezes. This means that ice has a lower density (mass per unit of volume) than liquid water. Thus ice floats on water. Without this property, lakes and streams in cold climates would freeze solid and lose most of their current forms of aquatic life. Because water expands upon freezing, it can also break pipes, crack a car's engine block (which is why we use antifreeze), break up streets and fracture rocks (thus forming soil). Water filters out wavelengths of ultraviolet radiation that would harm some aquatic organisms.
Water is the lifeblood of the biosphere. It connects us to one another, to other forms of life and to the entire planet.