 Determining the hardness of water
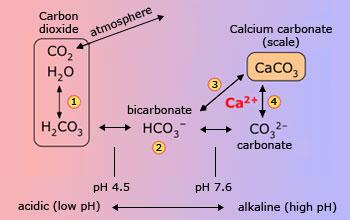
Carbon dioxide reacts with water to form carbonic acid (1) which at ordinary environmental pH exists
mostly as bicarbonate ion (2). Microscopic marine organisms take this up as carbonate (4) to form calcite skeletons which, over
millions of years, have built up extensive limestone deposits. Ground water, made slightly acidic by CO2 (both that
absorbed from the air and from the respiration of soil bacteria) dissolve the limestone (3), thereby acquiring calcium and
bicarbonate ions and becoming "hard". If the HCO3 concentration is sufficiently great, the combination of
processes (2) and (4) causes calcium carbonate ("lime scale") to precipitate out on surfaces such as the insides of
pipes. (Calcium bicarbonate itself does not form a solid, but always precipitates as CaCO3).
Determining the hardness of water
Carbon dioxide reacts with water to form carbonic acid (1) which at ordinary environmental pH exists
mostly as bicarbonate ion (2). Microscopic marine organisms take this up as carbonate (4) to form calcite skeletons which, over
millions of years, have built up extensive limestone deposits. Ground water, made slightly acidic by CO2 (both that
absorbed from the air and from the respiration of soil bacteria) dissolve the limestone (3), thereby acquiring calcium and
bicarbonate ions and becoming "hard". If the HCO3 concentration is sufficiently great, the combination of
processes (2) and (4) causes calcium carbonate ("lime scale") to precipitate out on surfaces such as the insides of
pipes. (Calcium bicarbonate itself does not form a solid, but always precipitates as CaCO3).
Water is of two types namely soft water and hard water. Soft water contains few or no calcium or magnesium metal cations. Soft water usually comes from peat or igneous rock sources, such as granite but may also derive from sandstone sources, since such sedimentary rocks are usually low in calcium and magnesium.
Hard water is the type of water that has high mineral content (in contrast with soft water). Hard water minerals primarily consist of calcium (Ca2+) and magnesium (Mg2+) metal cations and sometimes other dissolved compounds such as bicarbonates and sulfates.
The simplest way to determine the hardness of water is the lather/froth test: soap or toothpaste, when agitated, lathers easily in soft water but not in hard water. More exact measurements of hardness can be obtained through a wet titration. The total water ‘hardness’ (including both Ca2+ and Mg2+ ions) is read as parts per million (ppm) or weight/volume (mg/L) of calcium carbonate (CaCO3) in the water.
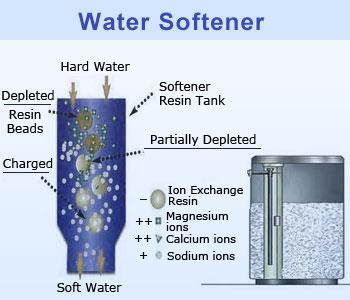 Temporary hardness can be removed by boiling but permanent hardness need to remove by cation resin exchange
process. This process is called water softening by water softener.
Temporary hardness can be removed by boiling but permanent hardness need to remove by cation resin exchange
process. This process is called water softening by water softener.
Types of hardness:
- Temporary hardness: Rain water is considered as the purest form of naturally occurring water, for the simple
reason, that it is formed by the evaporation and condensation of sea water. However, when the rain drops travel through
atmosphere, they absorb carbon dioxide gas. Thus, rain water is a very weak solution of carbonic acid.
When this rain water flows over or percolates through the rocks containing calcium carbonates, (such as lime stone, marble, chalk) or magnesium carbonate (magnesite or dolomite) it reacts with them, to form their respective soluble hydrogen carbonates, which render the water temporarily hard.
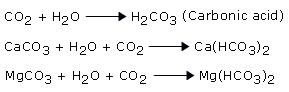
- Permanent hardness: In nature, following minerals are present in the form of rocks:
- Gypsum or calcium sulphate (CaSO4).
- Kieserite or magnesium sulphate (MgSO4).
- Kyanite or alumino silicate (Al2SiO5).
When ground water either flows over, or percolates through, the above mentioned minerals, it partly dissolves them and hence gets permanently hard in nature. However, all soluble salts of metals below calcium or magnesium in metal activity series can also react with soap solution to form scum, but these salts are not present in natural ground water and hence are not regarded as a cause of hardness. Sea water, on the other hand, practically contains dissolved salts of almost all the metals. Thus, sea water does not lather with soap solution. At the same time it cannot be called hard water because of following reasons.
- It is not a naturally occurring ground water.
- The scum may be formed by the ions other than calcium or magnesium.