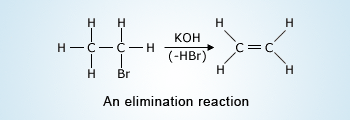
An elimination reaction is an organic reaction mechanism. In an elimination reaction two substituents are eliminated from a molecule in either a one or two–step mechanism.
Eliminations are the opposite of addition reactions. For an elimination to occur, it is necessary for the β–carbon to be bonded at least to one hydrogen.
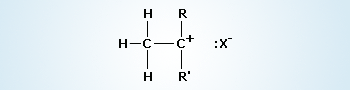 Formation of a carbocation intermediate (I step).
Formation of a carbocation intermediate (I step).
Uni molecular elimination reaction (E1 reaction):
It is a two step mechanism. This reaction mechanism is used to explain the formation of an alkenes from alkyl halides through a carbanion intermediate. It is regarded as first–order kinetics, because only one reactant molecule is involved in rate determining step. Hence it is called uni molecular elimination reaction.
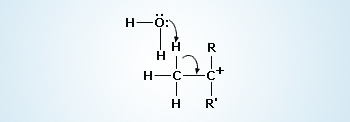 Deprotonation by a Nucleophile
Deprotonation by a Nucleophile
The first step in the E1 mechanism is the slow step and it is the rate–determining step. In the first step of the reaction, the compound ionizes as a carbocation and a halide ion. The halide ion separates from the compound carrying the electron pair with it, from the carbon–halide bond. It can sustain this negative charge due to its high electronegativity.
The first step in an E1 reaction is identical to the first step in an SN1 reaction. This leads to the formation a mixture of products. For example, treating tert–butyl chloride on reaction with aqueous ethanol at 25°C, gives substitution products in 83% yield and an elimination product (2–methylpropene) in 17% yield.
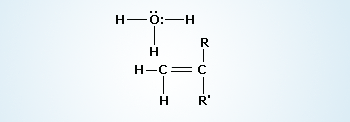 Formation of an Alkene(II step)
Formation of an Alkene(II step)
The spontaneous formation of the carbocation is the slow step in both reactions. Once formed, the carbocation reacts rapidly following either the SN1 or the E1 path.
The R groups should be substituents able to donate electron density to help stabilize the carbocation. Carbocation rapidly transfers a proton to a molecule of water, producing an alkene (double bond).
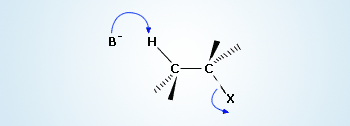 Abstraction of a β – hydrogen by a base
Abstraction of a β – hydrogen by a base
Biomolecular elimination reaction (E2 reaction):
E2 mechanism is a one–step process of elimination with a single transition state. These reactions are usually observed in primary or secondary substituted alkyl halides.
The rate of reaction is influenced by both the alkyl halide and the base. Hence it is known to be a Biomolecular elimination reaction.
When an alkyl halide is reacted with a suitable base, β–hydrogen bonded to the β–carbon of an alkyl halide is easily removed by the base because electron density in the molecule is pulled to the electronegative halide, leaving the β–hydrogen with a slight positive charge.
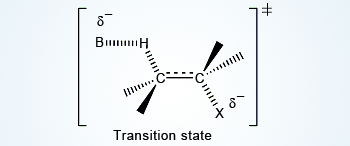
As the base abstracts, the hydrogen (proton) electron density is pushed through the system to expel the halide.
Partial bonds now exist throughout the system (H–C–C–X). The carbon–carbon bond is developing double bond character. As E2 mechanism results in formation of a pi bond, the two leaving groups (often a hydrogen and a halogen) should be in antiperiplanar in their transition state. An antiperiplanar transition state has staggered conformation with lower energy than a synperiplanar transition state which is in eclipsed conformation with higher energy. The reaction mechanism involving staggered conformation is more favorable for E2 reaction.
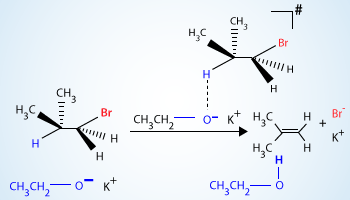 Elimination reaction of isobutylbromide with potassium ethoxide in ethanol.
Elimination reaction of isobutylbromide with potassium ethoxide in ethanol.
E2 reaction is a bimolecular reaction because two species are involved in the rate–limiting step.

The rate–limiting step in this reaction depends on the concentrations of the substrate and the base. If the concentration of one of these species is doubled the rate doubles.