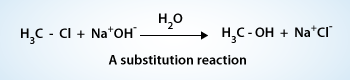
 Substitution reaction of an alkyl halide
In this reaction a hydroxide ion from sodium hydroxide replaces the chlorine of methyl chloride.
Substitution reaction of an alkyl halide
In this reaction a hydroxide ion from sodium hydroxide replaces the chlorine of methyl chloride.
Substitutions are the characteristic reactions of saturated compounds such as alkanes and alkyl halides, and of aromatic compounds (even though they are unsaturated).
In a substitution, one group replaces another.
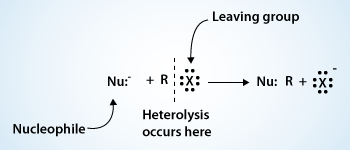 In nucleophilic substitution reactions the carbon–halogen bond of the alkyl halide substrate undergoes heterolysis and the unshared pair of the nucleophile is used to form a new bond to the carbon atom.
In nucleophilic substitution reactions the carbon–halogen bond of the alkyl halide substrate undergoes heterolysis and the unshared pair of the nucleophile is used to form a new bond to the carbon atom.
Nucleophilic substitution reactions:
In this type of reaction a nucleophile, a species with an unshared electron pair, reacts with a substrate and replaces the substituent (leaving group). As the substitution reaction is initiated by a nucleophile, it is called a nucleophilic substitution reaction.
Nucleophilic substitution reactions are more understandable and useful if we know something about their mechanisms, number of steps involved and the type of intermediates formed in the reaction. In order to know these, we need to know something about the rates of chemical reactions. The mechanism involved in SN1 and SN2 are discussed below.
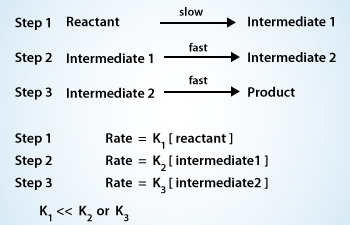 A multi–step reaction
When we say that the first step in this example is intrinsically slow. The rate constant(K) for step 1(Slow Step) is very much smaller than the rate constant for step 2 or for step 3
A multi–step reaction
When we say that the first step in this example is intrinsically slow. The rate constant(K) for step 1(Slow Step) is very much smaller than the rate constant for step 2 or for step 3
Unimolecular nucleophilic substitution reaction (SN1 Reaction):
The reaction of tert–butyl bromide with hydroxide ion:
When tert–butyl bromide reacts with sodium hydroxide in a mixture of water and acetone, tert–butyl alcohol is formed. The rate of formation of tert–butyl alcohol is dependent on the concentration of tert–butyl bromide, but it is independent of the concentration of hydroxide ion. Doubling the tert–butyl bromide concentration doubles the rate of the reaction, but changing the hydroxide ion concentration (within limits) has no appreciable effect. It is a multi–step reaction.
Multi–step reactions and the rate–determining step:
If a reaction takes place in a series of steps, and if one step is intrinsically slower than all the others, then the rate of the overall reaction will be essentially the same as the rate of this slow step. This slow step, consequently, is called the rate–limiting step or the rate–determining step.
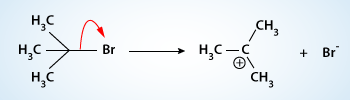
Mechanism of SN1 reaction:
The mechanism for the reaction of tert–butyl bromide with water apparently involves three steps. Two distinct intermediates are formed.
The first step is the slow step, it is the rate–determining step. In it a molecule of tert–butyl bromide ionizes and becomes a tert–butyl cation and a bromide ion as a result of heterolytic cleavage of carbon–bromine bond. Because no other bonds are formed in this step, it should be highly endothermic and it takes place largely because of the ionizing ability of the solvent, water. The carbocation is in the trigonal planar form and is therefore always achiral. Carbocation formation in general takes place slowly because it is usually a highly endothermic process.
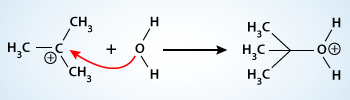
The positive charge is delocalized and therefore the nucleophile can approach the carbocation from either side. This will result in a racemic mixture of products if the carbon is a stereocenter. Here the nucleophile, oxygen of water molecule is not negatively charged but it has a lone pair of electrons.
In the second step the highly reactive tert–butyl cation intermediate reacts rapidly with water to produce a tert–butyloxonium ion (another intermediate). Now, the positive charge on the carbocation has been transferred to the nucleophile.
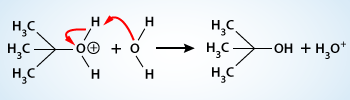
To neutralize the compound the hydrogen (proton) must be removed. This might be accomplished with the solvent or any other proton accepting species. Here tert–butyloxonium ion, in the third step, rapidly transfers a proton to a molecule of water producing tert–butyl alcohol.
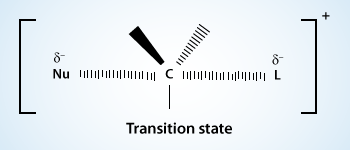
The important transition state for the SN1 reaction is the transition state of the rate determining step [TS(1)]. In it the carbon–bromine bond of tert–butyl bromide is largely broken and ions are beginning to develop. The solvent (water) stabilizes these developing ions by solvation.
The rate of formation of tert–butyl alcohol is dependent on the concentration of tert–butyl bromide.
Rate ∝ [(CH3)3CBr]
Rate = k[(CH3)3CBr]
In the above case, only tert–butyl bromides are involved in the rate determining step(Slow Step) as the hydroxide ions are not participating in the transition state - the step that controls the rate of the reaction. This reaction is said to be unimolecular (substrate only) substitution reaction.
 The rate of formation of methyl alcohol is dependent on concentrations of both methylchloride and base.
The rate of formation of methyl alcohol is dependent on concentrations of both methylchloride and base.
Biomolecular nucleophilic substitution reaction (SN2 reaction):
A bimolecular reaction, such as the SN2 reaction, is the one in which two reactant molecules take part in the transition state of the slow step or the rate–determining step of a reaction. For this reason, the concentrations of both the nucleophile and the alkyl halide are proportional to the observed SN2 reaction rate.
Kinetics of a nucleophilic substitution reaction:
The reaction that takes place between methyl chloride and hydroxide ion in aqueous solution under specific temperatures, show that the rate depends on the concentration of methyl chloride and on the concentration of hydroxide ion is an example of SN2 reaction. When we double the concentration of methyl chloride, the rate doubles. When we double the concentration of hydroxide ion, the rate doubles. When we double both concentrations, the rate increases by a factor of four.
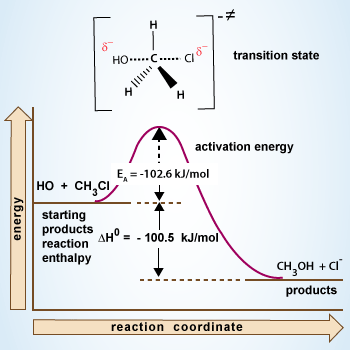 Reaction coordinate diagram for an SN2
Reaction coordinate diagram for an SN2
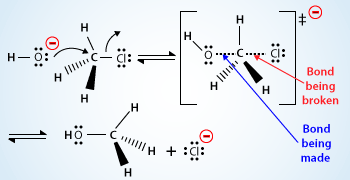
Mechanism for the SN2 reaction:
According to this mechanism, –OH the nucleophile, approaches the carbon bearing –Cl the leaving group.
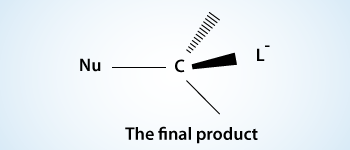
If the atom is being attacked (carbon atom) is a stereocenter there is an inversion of stereochemistry. The carbon atom begins to invert its configuration. The bond between the carbon and the nucleophile is partially formed and the bond between the carbon and the leaving group is partially broken.
As the reaction progresses, the bond between the nucleophile and the carbon atom strengthens and the bond between the carbon atom and the leaving group weakens. As this happens, the carbon atom has its configuration turned inside out (umbrella type inversion), it becomes inverted, and the leaving group is pushed away.
The formation of the bond between the nucleophile and the carbon atom provides most of the energy necessary to break the bond between the carbon atom and the leaving group. As the nucleophile is involved in the rate–determining step of SN2 reactions, stronger nucleophile reacts faster. Stronger nucleophiles are said to have an increased nucleophilicity.
There is no intermediate in an SN2 reaction, just a transition state is present. A transition state doesn't have a real lifetime, it is the highest energy point on the reaction coordinate as starting materials transition into products.
Rate ∝ [CH3Cl][OH−]
Rate = k[CH3Cl][OH−]
where (k) is called the rate constant.
For the reaction to take place, a hydroxide ion and a methyl chloride molecule must collide. Hence we say that the reaction is bimolecular (By bimolecular we mean that two species are involved in the step whose rate is being measured.). SN2 reaction is a second order reaction as the rate is dependent on two reactant molecules.