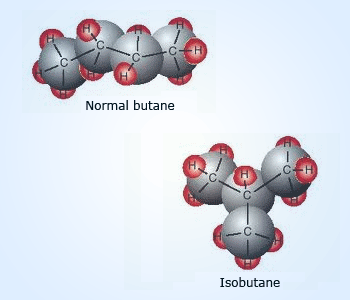
 Chain isomers
Chain isomers
The phenomenon of compounds having the same molecular formula but different structural formula is called Isomerism. These compounds are called Isomers. The Organic isomers are categorized as structural isomers and stereoisomers.
Structural isomerism:
Structural isomers are compounds with the same molecular formula but
with different physical properties, as the atoms are arranged differently. These include Chain isomerism, Position
isomerism, Functional isomerism, Metamerism and Tautomerism.
Chain isomerism is due to difference in the carbon chains of molecules with same molecular formula. For example, the molecular formula C4H10 gives two isomers, n–butane and 2–methylpropane (isobutene). These isomers remain part of the same organic family, although their boiling points differ considerably. Boiling point of n–butane is −0.5°C whereas that of isobutane is −11.7°C. The different shapes of the molecules mean that they fit together in different ways, which affects the strengths of the intermolecular forces.
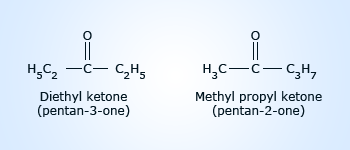 Metamers
Metamers
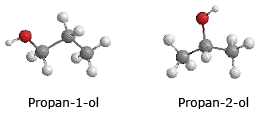
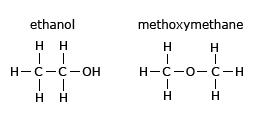 Position isomerism and Functional isomerism
Position isomerism and Functional isomerism
Position isomerism is due to difference in the position of an atom or group in a molecules with same molecular
formula.
Functional isomerism is due to difference in the nature of the functional group. For example, the two isomers of
C2H6O are members of different organic families – ethanol is an alcohol and methoxymethane (Dimethyl
ether) is an ether.
Metamerism is due to difference in nature of the alkyl groups attached to the same functional group.
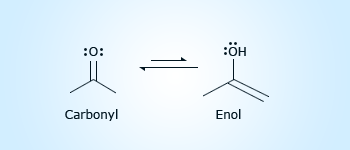 Tautomers
Tautomers
Tautomerism is inter convertible nature of the functional group due to the position of an atom in the molecule
Stereoisomerism
The three–dimensional arrangement of the bonds in a molecule allow different possible orientations in space. This results
in two different forms, and however hard you try you cannot superimpose the image of one onto the other. Two such forms of a
compound are called stereoisomers.
Two types of stereoisomerism are discussed.
1. Geometrical isomerism
2. Optical isomerism
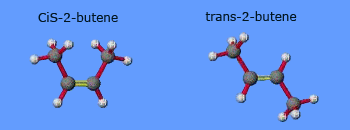 Geometrical isomers
Geometrical isomers
Geometrical isomerism :
Geometrical isomerism is one type of stereoisomerism. It arises due to the lack of free rotation of an atom or group around a
bond, frequently a double bond. Geometrical isomers occur when components of the molecule are arranged on different sides of the
molecule. Geometric isomers frequently exhibit different physical properties, and this type of isomerism can influence their
chemical reactions too.
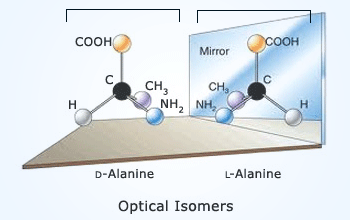 Optical isomers
Optical isomers
Optical isomerism :
Optical isomerism is another type of stereoisomerism. If two objects are the mirror image of each other and they are not
superimposable, then they are said to be chiral. Chirality is an exclusive property of asymmetrical objects,
and organic molecules are no exception. The non–systematic isomers like this that are non superimposed mirror images of
each other are called enantiomers or optical isomers . These enantiomers have an effect on plane–polarized light (Polarized
light is produced by passing light through a polarizing material which causes the light to oscillate in only one plane).
One enantiomer will rotate the polarized light to the right (clockwise), indicated by (+), and the other
will rotate it to the left (counter clockwise), indicated by (−). A substance that rotates plane–polarized light
in the clockwise direction is also said to be dextrorotatory (D), and one that rotates plane–polarized
light in a counter clockwise direction is said to be levorotatory (L).