 Cycloalkane
Cycloalkane
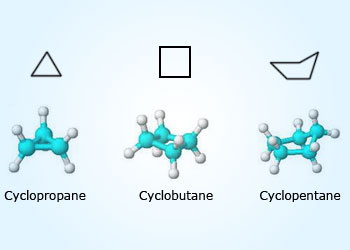
The Cycloalkanes are a family of Saturated hydrocarbons. The hydrocarbon with saturated rings are cycloalkane. These cycloalkanes have the general formula CnH2n instead of CnH2n+2 for the chain molecules.
Cycloalkanes behave very similar to the other alkanes, but they tend to have higher melting and boiling points. They have the same name as the corresponding straight chain molecule, but with the prefix ’cyclo –‘. These ring molecules are not aromatic compounds since the aromatic ring structure is based on the benzene ring.
Reactivity of cycloalkanes:
Just as in the straight–chain alkanes, the four orbitals around each carbon atom in the cycloalkanes are arranged
tetrahedrally. However, the angle between the carbon atoms in cyclopropane is 60°, which is considerably less than the
tetrahedral angle of 109.5°. This leads to much weaker C–C bonds in cyclopropane than in straight–chain propane,
since there is much less overlap possible between the orbitals of the carbon atoms in cyclopropane than in propane.
The C–C bonds in cyclopropane are very strained compared to straight–chain propane. In order to relieve this
tremendous angle strain cyclopropanes engage in high reactivity than straight–chain propane. This effect is less pronounced
in cyclobutane, which has a bond angle of 90°, Cyclobutane forms a square molecule, which is less reactive than cyclopropane,
but is more reactive than butane. Cyclopentane and higher cycloalkanes have a similar reactivity to their straight–chain
equivalents, the bonds in cyclopentane are almost entirely free from strain. Cycloalkanes with six or more carbon atoms are able
to relieve the strain by puckering the ring.
 Chair conformation of Cyclohexane
Chair conformation of Cyclohexane
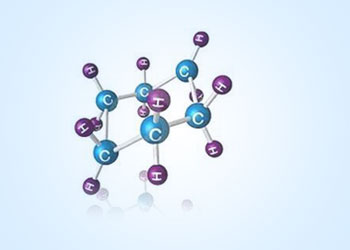
Chair structure of cyclohexane:
Cyclohexane has many conformers among them the chair structure of cyclohexane is considered to be the “perfect”
conformation. The chair conformer of cyclohexane gives relief from angle strain and steric strain. Cyclohexane consists of a
six–membered ring structure in which every C–C bond exists in a staggered conformation. So there is no torsional
strain and angle strain. Since it overcomes these strains cyclohexane is the most stable compound in all cyclo alkanes.