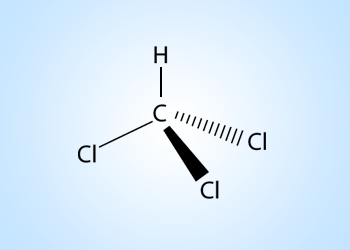
 Chloroform
Chloroform
The substitution of a chlorine atom into a molecule of the organic family known as the alkanes results in a compound with anesthetic properties. The compound is called chloroform (CHCl3) or trichloromethane.
Increasing the number of chlorine atoms in the compound increases the depth of anesthesia given. But unfortunately it also increases the toxicity of the compound.
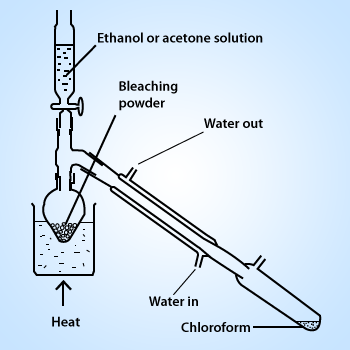 Preparation of Chloroform
Preparation of Chloroform
Preparation of Chloroform(trichloromethane):
Chloroform is prepared in the laboratory
as well as on the industrial scale by heating ethyl alcohol or acetone with bleaching powder or chlorine and alkali.
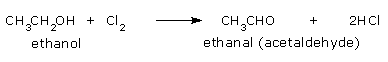
From ethanol: The formation of chloroform from ethanol takes place as follows:
Oxidation of ethyl alcohol by chlorine
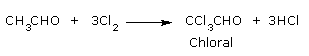
Formation of chloral by the action of chlorine on acetaldehyde so formed.
Hydrolysis of chloral to chloroform by calcium hydroxide or alkali (NaOH).
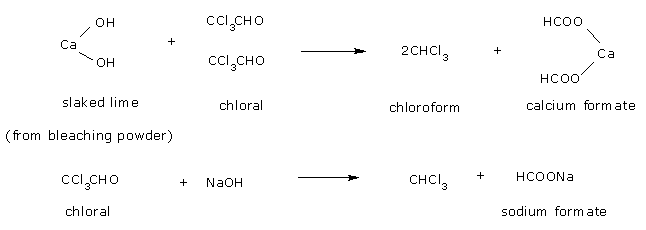
The net reaction when bleaching powder is used is,
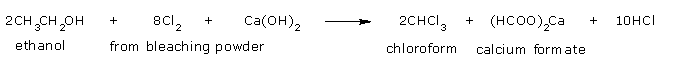
Preparation of pure chloroform:
Pure chloroform needed for medicinal use is obtained by heating chloral hydrate with concentrated solution of NaOH.
 Tear gas used for riot control.
Tear gas used for riot control.
Uses of chloroform:
Chloroform is used,
- As an anesthetic. It has now been replaced.
- As a solvent for fats, waxes and resins.
- As a preservative for anatomical specimen.
- For the preparation of chloretone, chloropicrin etc.,
- As a source for the production of salicyaldehyde, freon, tear gas, chloretone etc.