 Preparation of soaps
Preparation of soaps
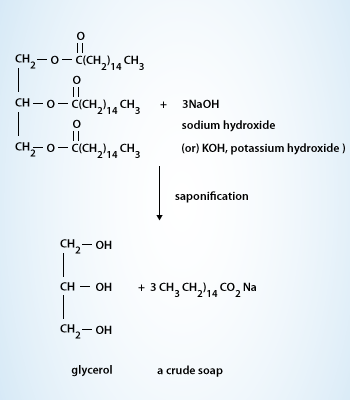
A soap is a salt of a fatty acid. Soaps are mainly used as surfactants for washing, bathing and cleaning, but they are also used in textile spinning and are important components of lubricants. Soaps for cleansing are obtained by treating vegetable or animal oils and fats with a strongly alkaline solution such as sodium hydroxide this process is called saponification. In saponification, the fats are first hydrolyzed into free fatty acids, which then combine with the alkali to form crude soap. Once the saponification reaction is complete, sodium chloride is added to precipitate the soap. The water layer is drawn off the top of the mixture and the glycerol is recovered using vacuum distillation. The crude soap obtained from the saponification reaction contains sodium chloride, sodium hydroxide and glycerol. These impurities are removed by boiling the crude soap curds in water and re-precipitating the soap with salt. After the purification process is repeated several times, the soap may be used as an inexpensive industrial cleanser. Sand or pumice may be added to produce a scouring soap. Other treatments may result in laundry, cosmetic, liquid, and other soaps.
Glycerol, often called glycerine, is liberated and is either left in or washed out and recovered as a useful by–product according to the process employed.
 A Micelle structure with hydrophilic head and hydrophobic tail.
A Micelle structure with hydrophilic head and hydrophobic tail.
How soaps clean:
Soaps are sodium or potassium salts of fatty acids, Each soap molecule has a long hydrocarbon chain, sometimes called its ‘tail’
and with a hydrophilic negatively–charged carboxylate ‘head’. In water, the sodium or potassium ions float free,
leaving a negatively–charged head. Soap is an excellent cleanser because of its ability to act as an emulsifying agent. This means
that while oil (which attracts dirt) doesn't naturally mix with water, soap can suspend oil/dirt in such a way that it can be removed.
The organic part of a natural soap is polar molecule. Its (water–loving) carboxylate group (–CO2) interacts with water
molecules via ion–dipole interactions and hydrogen bonding. The hydrophobic part of a soap molecule, its long, non–polar
hydrocarbon chain, does not interact with water molecules.
The hydrocarbon chains are attracted to each other by dispersion forces and cluster together, forming structures called micelles. In these micelles, the carboxylate groups form a negatively–charged spherical surface, with the hydrocarbon chains inside the sphere. Because they are negatively charged, soap micelles repel each other and remain dispersed in water. Grease and oil are non– polar and insoluble in water. When soap and soiling oils are mixed, the non–polar hydrocarbon portion of the micelles break up the non–polar oil molecules. A different type of micelle then forms, with non–polar soiling molecules in the center. Thus, grease and oil and the ‘dirt’ attached to them are caught inside the micelle and can be rinsed away.