 Three major allotropes of Sulfur; Rhombic, Monoclinic and Plastic
Three major allotropes of Sulfur; Rhombic, Monoclinic and Plastic
Sulfur is a solid at room temperature and 1 atm pressure. It is the sixteenth most abundant element in Earth's crust. It exists in a variety of forms naturally, including elemental sulfur, sulfides, sulfates, and organosulfur compounds. For a very long time sulfur has been mined using the Frasch process however in the modern age with a growing concern over sulfur emissions from industrial processes. Sulfur produced from this process is used in a variety of ways including in vulcanizing rubber and as fungicide to protect grapes and strawberries.
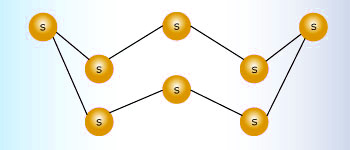 The puckered ring structure of S8 molecule
The puckered ring structure of S8 molecule
sulfur is very unique in its ability to form a wide range of allotropes, more than any other element in the periodic table. The most common state for sulfur to be in is the solid S8 ring. sulfur exists in the gaseous form in five different forms (S, S2, S6, and S8). In order for sulfur to get to these states one must apply a sufficient amount of heat.
Two very common oxides of sulfur are sulfur dioxide (SO2) and sulfur trioxide(SO3).
These two compounds are used in the production of sulfuric acid, which can be used in a variety of reactions. Sulfuric acid is one of the top manufactured chemicals in the US, and is primarily used in the manufacture of fertilizers.
Sulfur also exhibits a wide range of oxidation states, with values ranging from –2 to +6. It is often the central ion in a compound and can easily hold up to 6 atoms around itself. When in the presence of hydrogen it forms the compound H2S which is a poisonous gas, without hydrogen bonds and a very small dipole moment. This reaction with hydrogen epitomizes how different oxygen and sulfur act despite their common valence electron configuration and common non–metallic properties.