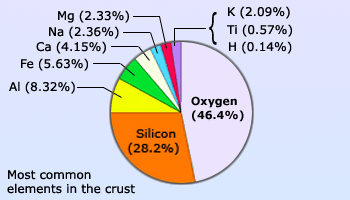
 Abundance of Oxygen in earth's crust
Abundance of Oxygen in earth's crust
Oxygen is a colorless, odorless and tasteless gas at room temperature and at 1 atm pressure. When in its liquid form it has a bluish color. It is the most abundant element in the Earth's crust and the most abundant element in sea water. It is second to nitrogen as the most abundant in the atmosphere.
There are many commercial uses for oxygen gas, which is typically obtained through the process of fractional distillation. It is used in the manufacture of iron, steel, and other chemical manufacturing. It is also used in water treatment, as an oxidizer in rocket fuel, for medicinal purposes, and in petroleum refining.
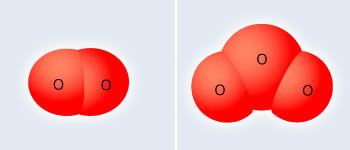 Two allotropes of Oxygen
Two allotropes of Oxygen
It exists as two allotropes: O2 and O3. The later allotropy, ozone, is a very good oxidizing agent and can be used as a substitute for chlorine in purifying drinking water without giving the water an odd taste. However, because of its unstable nature it disappears and leaves the water unprotected from bacteria. Ozone at very high altitudes in the atmosphere is responsible for protecting the Earth's surface from ultraviolet radiation; however, at lower altitudes it becomes a major component of smog.
Its primary oxidation states are –2, –1, 0 and –1/2 in O2–. When oxygen reacts with metals, it forms oxides that are mostly ionic in nature. It is rarely featured as the central atom in a structure and can never have more than 4 elements bonded to it due to its small size and its inability to create an expanded valence shell. When it reacts with hydrogen, it forms water, which is extensively hydrogen bonded, has a large dipole moment and is considered an universal solvent.