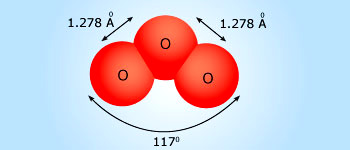
 Structure of the O3 molecule
Structure of the O3 molecule
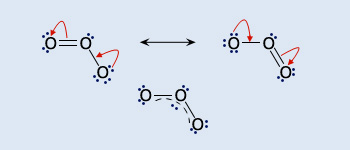 The resonance forms of ozone
The 'averaged' structure of ozone showing the de-localized molecular orbital.
The resonance forms of ozone
The 'averaged' structure of ozone showing the de-localized molecular orbital.
Ozone is a less stable allotrope of oxygen, it is a pale–blue poisonous gas with a sharp, irritating odor. Most people can detect about 0.01 ppm in air. Exposure to 0.1 to 1ppm produces headaches, burning eyes, and irritation to the respiratory passages.
Structure of the O3 molecule
The molecule possesses a π bond that is de-localized over the three oxygen atoms. The molecule dissociates readily forming reactive oxygen atoms.
O3(g)  O2(g) + O(g) ΔH0 = 105 kJ
O2(g) + O(g) ΔH0 = 105 kJ
Ozone is a stronger oxidizing agent than di-oxygen. One measure of this oxidizing power is the high standard reduction potential of O3, compared to that of O2.
O3(g) + 2H+(aq) + 2e–  O2(g) + H2O(l) E0 = 2.07V
O2(g) + H2O(l) E0 = 2.07V
O2(g) + 4H+(aq) + 4e–  2H2O(l) E0=1.23V
2H2O(l) E0=1.23V
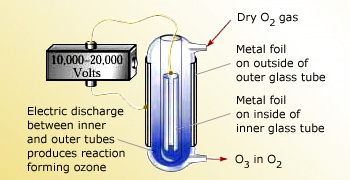 Preparation of Ozone (O3)
Preparation of Ozone (O3)
Standard reduction potential values
Ozone forms oxides with many elements under conditions where O2 will not react; indeed, it oxidizes all of the common metals except gold and platinum.
Ozone can be prepared by passing electricity through dry O2:
3O2(g) 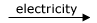 2O3(g) ΔH0 = 285kJ
2O3(g) ΔH0 = 285kJ
O3 can be prepared in an apparatus.
The gas cannot be stored for long, except at low temperature, because it readily decomposes to O2. The decomposition is catalyzed by certain metals, such as Ag, Pt, and Pd, and by many transition metal oxides.
Ozone is sometimes used in treatment of domestic water in place of chlorine. Like Cl2, it serves to kill bacteria and oxidize organic compounds. The largest use of ozone, however, is in the preparation of pharmaceuticals, synthetic lubricants, and other commercially useful organic compounds, where O3 is used to serve carbon–carbon double bonds.
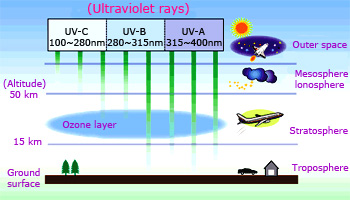 Ozone layer absorbs harmful UV - rays in stratosphere.
Ozone layer absorbs harmful UV - rays in stratosphere.
Ozone is an important component of the upper atmosphere, where it serves to screen out ultraviolet radiation. In this way ozone protects earth from the effects of these high–energy rays. For this reason, depletion of stratospheric ozone is a major scientific concern. However, in the lower atmosphere, ozone is considered an air pollutant. It is a major constituent of smog. Because of its oxidizing power, it causes damage to living systems and structural materials, especially rubber.
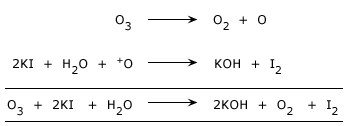
 Potassium iodide (KI) to Iodine(I2)
Potassium iodide (KI) to Iodine(I2)
Reactions of ozone:
It liberates iodine from a solution of potassium iodide.
It oxidizes sulphur dioxide to sulphur trioxide.
3SO2(g) + O3(g)  3SO3(g)
3SO3(g)
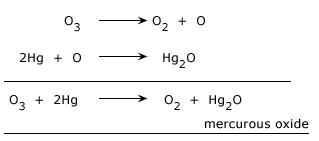
 Tailing of Mercury
It is one of the test to protect the Ozone. When ozone is passed through mercury, it loses its meniscus and sticks to the glass due to the formation of mercurous oxide. This is called tailing of mercury. The meniscus can be restored by shaking it with water
Tailing of Mercury
It is one of the test to protect the Ozone. When ozone is passed through mercury, it loses its meniscus and sticks to the glass due to the formation of mercurous oxide. This is called tailing of mercury. The meniscus can be restored by shaking it with water
With mercury: It oxidizes Mercury (0) into Mercuric (II) oxide.
Bleaching action: Due to its oxidising nature, ozone acts as a good bleaching agent for vegetable colouring matter.
Vegetable colouring matter + O3  Colourless matter + O2
Colourless matter + O2
Reducing property: Ozone reduces peroxides to oxides and in turn gets reduced to oxygen. For example, with H2O2 and BaO2, it gives respectively H2O and BaO.
H2O2 + O3  H2O + 2O2
H2O + 2O2
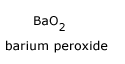 + O3
+ O3  BaO + 2O2
BaO + 2O2
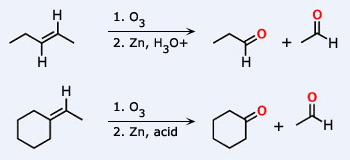 Addition reactions of Ozone
Addition reactions of Ozone
Addition reactions: Ozone with unsaturated organic compounds containing double bond forms addition products called ozonides.
These ozonides are decomposed by water or dilute acids giving aldehydes and hydrogen peroxide in most of the cases.
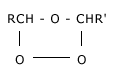 + H2O
+ H2O  RCHO + R–CHO + H2O2
RCHO + R–CHO + H2O2
This reaction forms the basis of locating the position of the double bond in the original unsaturated molecule from the nature of the products formed. This reaction is termed ozonolysis.