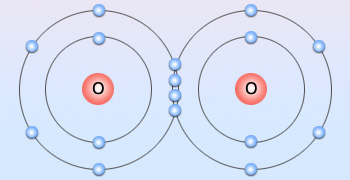
 Diatomic oxygen
Two pairs of electrons shared between two oxygen atoms forming a double bond
Diatomic oxygen
Two pairs of electrons shared between two oxygen atoms forming a double bond
 Joseph Priestly, FRS (13 March 1733 - 6 February 1804)
Joseph Priestley was an 18th-century English theologian, Dissenting clergyman, natural philosopher, chemist, educator, and political theorist who published over 150 works. He is usually credited with the discovery of oxygen, having isolated it in its gaseous state, although Carl Wilhelm Scheele and Antoine Lavoisier also have a claim to the discovery.
Joseph Priestly, FRS (13 March 1733 - 6 February 1804)
Joseph Priestley was an 18th-century English theologian, Dissenting clergyman, natural philosopher, chemist, educator, and political theorist who published over 150 works. He is usually credited with the discovery of oxygen, having isolated it in its gaseous state, although Carl Wilhelm Scheele and Antoine Lavoisier also have a claim to the discovery.
At room temperature dioxygen is a colorless and odorless gas. It condenses to a liquid at –183°C. It is only slightly soluble in water, but its presence in water is essential to marine life. Dioxygen gas constitutes 20.8% of the volume of air.
The electron configuration of the oxygen atom is [He]2s22p4
Thus, oxygen can complete its octet of electrons either by picking up two electrons to form the oxide ion, O2−, or by sharing two electrons. In its covalent compounds it tends to form two bonds: either as two single bonds, as in H2O, or as a double bond, as in formaldehyde, H2C=O. The O2 molecule itself contains a double bond.
The bond in oxygen is very strong (the bond enthalpy is 495kJ/mol). Oxygen also forms strong bonds with many other elements. Consequently, many oxygen containing compounds are thermodynamically more stable than O2. At certain conditions O2 rapidly accelerates , producing a reaction of explosive violence.
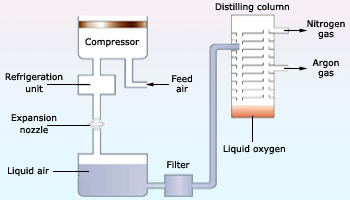 Industrial production of oxygen by fractional distillation of liquefied air
Industrial production of oxygen by fractional distillation of liquefied air
Preparation of Oxygen:
Nearly all commercial oxygen is obtained from air. The normal boiling point of O2 is –183°C, whereas that of N2, the other principal component of air, is –196°C. Thus, when air is liquefied and then allowed to warm, the N2 boils off, leaving liquid O2 contaminated mainly by small amounts of N2 and Ar.
A common laboratory method for preparing O2 is the thermal decomposition of potassium chlorate, KClO3, with manganese dioxide, MnO2, added as a catalyst.
2KClO3(s)  2KCl(s) + 3O2(g)
2KCl(s) + 3O2(g)
Like H2, O2 can be collected by displacement of water because of its relatively low solubility.
Much of the O2 in the atmosphere is replenished through the process of photosynthesis by plants, in which green plants use the energy of sunlight to generate O2, but also uses up CO2.
Uses of Oxygen:
Oxygen is the one of the most widely used industrial chemicals, ranking behind only sulfuric acid, H2SO4, and nitrogen N2. About 2.7 × 1010 kg (30 million tons) of O2 is used annually in the United states. Oxygen can be shipped and stored either as a liquid or in steel containers as a compressed gas. However, about 70 percent of the O2 output is generated where it is needed.
Oxygen is by far the most widely used oxidizing agent. Over half of the O2 produced is used in the steel industry, mainly to remove impurities from steel. It is also used to bleach pulp and paper (Oxidation of colored compounds often gives colorless products).
In medicine, oxygen eases breathing difficulties. It is also used together with acetylene, C2H2, in oxyacetylene welding.