 Metal used in construction of a tower
The strength of the tower depends on the metal or the alloy used in construction. An alloy is a mixture
of two or more metals.
Metal used in construction of a tower
The strength of the tower depends on the metal or the alloy used in construction. An alloy is a mixture
of two or more metals.
A metal can be an element, compound or alloy that is a good conductor of both electricity and heat. Metals are usually malleable, ductile and shiny. The most abundant metal in earth's crust is aluminum.
The metals have free unbound electrons, beyond closed shells. In metals, these unbound electrons are given off or donated during compound formation. Metals are shown on the left – hand side and centre of the periodic table.
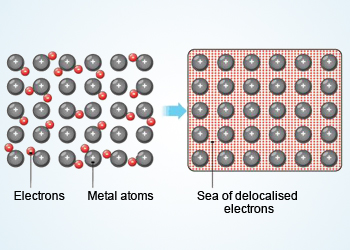 Metallic bonding
Metallic bonding
Metallic Bond:
Metal atoms are held together by electrostatic forces, joined in a particular way. The outer electrons of most metal atoms tend
to be weakly held to the atomic nucleus. Consequently, these outer electrons are easily dislodged, leaving behind positively
charged metal ions.
Many electrons easily dislodged from a large group of metal atom flow freely through the resulting metal ion grid. This "fluid" of electrons holds the positively charged metal ions together in the type of chemical bond known as a metallic bond.
These loose electrons form a kind of "electronic fluid" that flows through the lattice of positively charged ion. The mobility of electrons in a metal accounts for the metal's significant ability to conduct electricity and heat. Also, metals are opaque and shiny because the free electrons easily vibrate to the oscillations of any light falling on them, reflecting most of it.
 Liquid metal
Liquid metal
 Metals have the ability to withstand hammering and can be made into thin sheets known as foils
this property is known as Malleability.
Metals have the ability to withstand hammering and can be made into thin sheets known as foils
this property is known as Malleability.
Physical properties:
Metals are solids at room temperature with the exception of mercury and gallium, which are liquids at room temperature. Gallium
melts at 85 degrees Fahrenheit and does not occur in nature. At low temperatures, gallium is a brittle solid.
All metals are hard(except sodium and potassium) and they have properties such as metallic lustre, malleability and ductility. Metal shave high densities, melting and boiling points and are good conductors of heat and electricity. Examples for the above discussed properties are as follow:
- Gold and silver metals are some of the best malleable metals.
- Gold is the most ductile metal. Copper and aluminum metals are also very ductile and can be drawn into thin copper wires and aluminum wires.
- Iron, copper, aluminum etc., are very hard metals. They cannot be cut with a knife.
- Silver and copper are the two best conductors of heat and electricity. Lead is the poorest conductor of heat. Bismuth, mercury and iron are also poor conductors.
- Tungsten metal has the highest melting point in the periodic table.
- Iridium and osmium metals have the highest densities. Sodium and potassium metals have low densities.
Metals have high electropositive character as a result can act as a reducing agent.
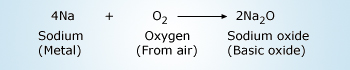 Sodium reacts with oxygen or air at ordinary room temperature. The resultant is sodium oxide.Sodium
is a highly reactive metal and catches fire easily when exposed to air.
Sodium reacts with oxygen or air at ordinary room temperature. The resultant is sodium oxide.Sodium
is a highly reactive metal and catches fire easily when exposed to air.
Chemical properties:
All metals react with oxygen to form basic metal oxides, these oxides dissolved in water forms alkaline solution. A single metal
can form various oxides depending on the valence state of the metal atom. For example Fe can form oxides FeO,
Fe2O3, Fe3O4. etc.
All metals on reaction with water form metal oxides and metal hydroxides. Hydrogen gas is released in this reaction. There are some metals that do not react with water at all, example zinc.
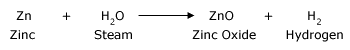
Zinc metal reacts with steam. Zinc forms ZnO (zinc oxide) and hydrogen are formed.
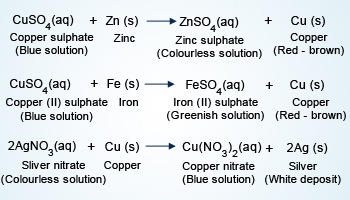 Metal displacement reactions
Metal displacement reactions
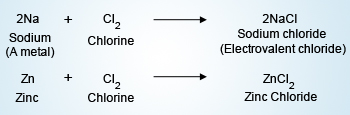 Reactions of metals with Chlorine
Na, Ca react readily with Cl to form NaCl and CaCl2. Mg reacts with Cl on heating to form
MgCl2. Zn forms a chloride easily. Fe and Cu need to be heated to form their chlorides.
Reactions of metals with Chlorine
Na, Ca react readily with Cl to form NaCl and CaCl2. Mg reacts with Cl on heating to form
MgCl2. Zn forms a chloride easily. Fe and Cu need to be heated to form their chlorides.
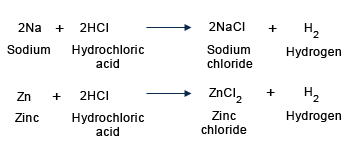
Most metals react with dilute acids by replacing hydrogen and forming a salt. Hydrogen gas is released. Metals like Cu, Ag, Au, Pt, Hg, do not react with dilute acids.
More reactive metal displaces the less reactive metal, when reacted with the metal salt or metal oxide. These are called metal displacement reactions.
When CuO is heated with Mg, MgO is formed and Cu is released. This is because Mg is more reactive than Cu. This reaction is irreversible, as Cu will never be able to displace Mg from MgO.

At suitable conditions metals react with Chlorine forms metal chlorides and metal hydrides are formed on reaction of metals with hydrogen.

Na, K, Mg and Ca react with H to form metal–hydrides. Other metals do not react with H easily.
The applications of metals include their use in building and bridge constructions,chassis of cars and
aeroplanes, tools, pipes, rail and road tracks,home appliances, electrical cables, jewelry etc.
Radioactive metals such as uranium and plutonium are used in nuclear power plants to produce energy via nuclear fission.