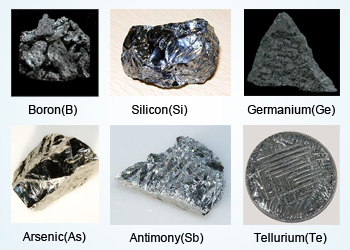
Metalloids are the elements which possess intermediate (Physical and chemical) properties to that of metals as well as non metals, hence they are also known as semi metals.
Metalloids in the periodic table are placed between the metals and the non metals. There are seven metalloids
in total. These seven metalloid are:
Boron(B), Silicon(Si), Germanium(Ge), Arsenic(As), Antimony(Sb), Tellurium(Te) and Selenium (Se).
Silicon is the second most abundant element in the Earth's crust.
 Allotropes of Selenium
Allotropes of Selenium
Carbon, aluminum, selenium, polonium and astatine are also less commonly known Metalloids. Metalloids exhibit allotropism.
Metalloids are dull and brittle solids in nature like non–metals but can conduct electricity like metals. They behave as non metals when they react with metals and behave as metals when they react with non–metals. Metalloids mostly behave as (weak) non–metals. They have intermediate ionization energies and electronegativity values and are amphoteric (weakly acidic) oxides.
Metalloids and their compounds have their common use in glasses, alloys, fire retardants, semiconductors, electronics, optical storage media and also in medicines. The metalloides have both toxic and medicinal properties. Metalloids have a key role in modern industry. Silicon and germanium metalloids are used as a semiconductors because they allow a controlled flow of electricity through them which is a useful property.
 Silicon and Germanium semiconductor
Silicon and Germanium semiconductor
Semiconductors have become very essential aspect of modern life due to their importance in electronics.
Semiconductors, especially silicon, has its use in radios, computers, televisions, telephones and computer chips.
The metalloids are used in making special types of glasses. Boron glass is used by aerospace engineers for space capsules and
other parts of space vehicles. Boron silicate glass test tubes, beakers and other important shatterproof glass containers are
find used by researchers and industry scientists.
Antimony is used in a wide range of fire proofing products due to the insulating property (Similar to Non–metals) of metalloids. Antimony trioxide is a flame–resistant compound that can be applied easily to a large range of products. Antimony makes car seats, clothing and many other products flame–retardant.
The metalloid arsenic is used in pesticide for farming. Arsenic intake is poisonous to humans.