 Colorless crystals of XeF2
Colorless crystals of XeF2
It is a Compounds of Xenon with Fluorine.In this compound, Xe appears in +2 oxidation state.
Preparation: It is prepared in the laboratory by heating xenon and fluorine in the molar ratio 1:3 in a nickel vessel at 400°C. The reaction products are quenched at –50°C. XeF2 is isolated by vacuum sublimation.
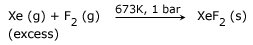
It can also be obtained by reacting together Xe and O2F2 (oxygen difluoride) at –118°C
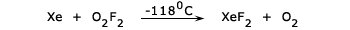
Physical properties:
- It is a colorless crystalline solid, and can be sublimed at room temperature.
- It melts at 303 K.
- It is soluble in HF
XeF2 is a strong oxidising and fluorinating agent.
 Structure and bonding
Structure and bonding
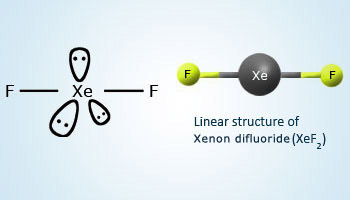
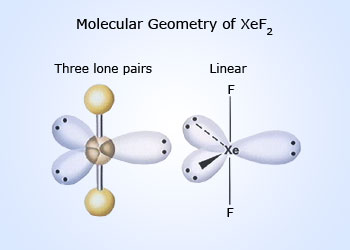
XeF2 is a linear molecule, F–Xe–F. Valence bond representation of XeF2 may be explained, if one of the 5p electrons is promoted to the 5d orbital.
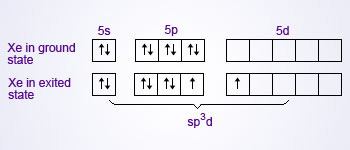
One 5s, three 5p and one 5d atomic orbitals hybridise to give five sp3d hybridised orbitals. The two orbitals, which are singly occupied are used by two fluorine atoms to form bonds with xenon and the rest of the three are occupied by the lone pairs
Chemical properties:
It undergoes substitution reaction when reacted with strong protonic acids such as, HSO3F, HClO4 and CF3COOH. In the case of HSO3F, the reaction is,
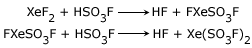 Similar reactions are possible with HClO4 and CF3COOH.
Similar reactions are possible with HClO4 and CF3COOH.
It oxidises iodine in the presence of fluoride ion acceptor to give IF.
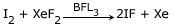
It reacts with NO to give nitrosyl fluoride.
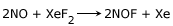
It reacts with sulphur trioxide.

Hydrogen reduces XeF2 to Xe.

With fluorine it gives higher fluoride.

It undergoes slow hydrolysis, when dissolved in water.

It reacts with SbF5 and AsF5 to form coordination complexes in which XeF2 acts as a donor of F −.
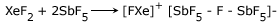
It fluorinates ethylene to give 1,1–difluoroethane.
