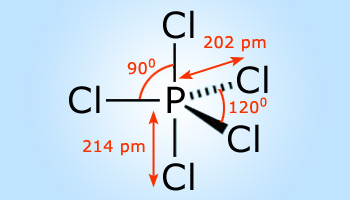
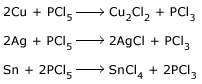 Phosphorus penta chloride with trigonal bipyramidal structure
Phosphorus penta chloride with trigonal bipyramidal structure
Phosphorus penta chloride is one of the important compounds of phosphorus and chlorine.
Preparation:
Phosphorus Penta chloride is prepared by passing an excess of dry chlorine into liquid trichloride. Chlorine reacts with phosphorus trichloride and solid phosphorus penta chloride is formed.
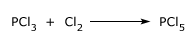
It may also be prepared by passing chlorine into a well cooled solution of phosphorus in carbondisulphide and crystallizing out of product formed.
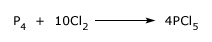
 Pale yellow crystals of PCl5
Pale yellow crystals of PCl5
General properties:
- Phosphorus penta chloride is a pale yellow crystalline solid having a pungent smell.
- It strongly fumes in moist air as it has great affinity for water.
- It sublimes at 100°C and the vapor when heated dissociate into phosphorus trichloride and chlorine.
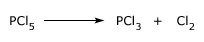
 Reaction of PCl5 with Water
Reaction of PCl5 with Water
 Phosphoryl chloride
Phosphoryl chloride
Action of water:
It is decomposed by water in two stages forming phosphorusoxychloride, POCl3, and phosphoric acid H3PO4 respectively.
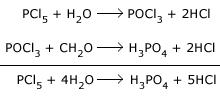
Action with hydroxy compounds: It reacts with organic compounds containing the hydroxy group.
Example: acids, alcohols etc.
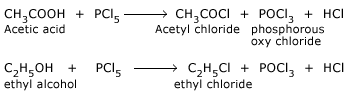
The reaction takes place with almost explosive violence.
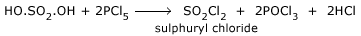
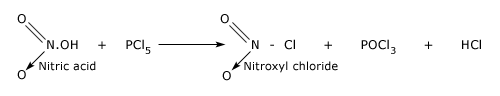
 Reaction of PCl5 with silver.
PCl5 is used for detecting the OH group in organic substances and replacing it by Cl.
Reaction of PCl5 with silver.
PCl5 is used for detecting the OH group in organic substances and replacing it by Cl.
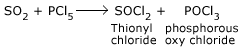
Reaction with SO2:
PCl5 combines with SO2 to form thionyl chloride.
Action with metals: Finely divided metals react with PCl5 to give corresponding chloride.
Example: