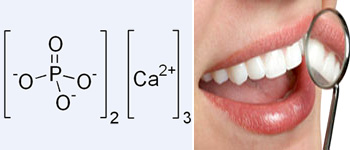
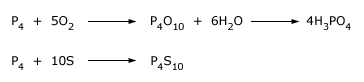 Tri calcium phosphate is the major composition of teeth and bones.
Tri calcium phosphate is the major composition of teeth and bones.
Phosphorus is the eleventh most abundant element in the earth's crust. Phosphorus is essential for life, both as a structural material in higher animals, and in the essential metabolism of both plants and animals. About 60% of bones and teeth comprise of Ca3(PO4)2 or [3(Ca3(PO4)2). CaF2], and an average person has 8lbs (3.5kg) of calcium phosphate in his body.
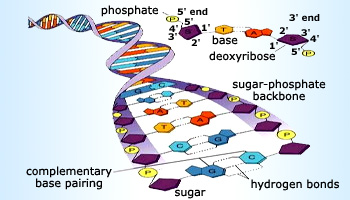 Sugar phosphate backbone in DNA
Sugar phosphate backbone in DNA
Nucleic acids such as DNA and RNA contain the genetic material for each cell. These nucleic acids are made up of polyester chains of phosphates and sugars with organic bases (adenine, cytosine, thymine and guanine). Phosphorus, in the form of adenosine triphosphate ATP and adenosine diphosphate ADP, is of vital importance for the production of energy in cells. When water splits a phosphate group of ATP, forming ADP an energy of 33KJ mol–1 is released.
 Phosphate rock and Chlorapatite
Phosphate rock and Chlorapatite
World production of phosphate rock was 145 million tonnes in 1992 (USA 32%, China 18%, Soviet union 15%, and Morocco 13%). Most is mined as fluoroapatite [3Ca3(PO4)2.CaF2], but some is found as hydroxy apatite [3Ca3(PO4)2.Ca(OH)2] and chloroapatite [3Ca3(PO4)2.CaCl2].
About 90% of phosphate rock is used directly to make fertilizers, and the remainder is used to make phosphorus and phosphoric acid.
 P4S3 is used to make match sticks
P4S3 is used to make match sticks
Elemental phosphorus is obtained by the reduction of calcium phosphate with C in an electric furnace at 1400–1500o C. Sand (silica SiO2) is added to remove the calcium as a fluid slag (calcium silicate) and to drive off the phosphorus as P4O10. The P4O10 is reduced to phosphorus by C. At this temperature gaseous phosphorus distills off, mainly as P4 but with some P2. This is condensed to white phosphorus P4 by passing the gas through water.
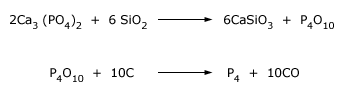
About 85% of the elemental Phosphorous produced is used to make very pure phosphoric acid H3PO4 and about 10% used to make P4S10 and P4S3 (which is used to make matches).