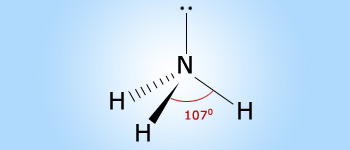
 Ammonia has a pyramidal shape with the central nitrogen element in sp3 hybrid state,
the H–N–H Bond angle in NH3 is 106° 45'. This is due to greater repulsion between the two
bond pairs of electrons.
Ammonia has a pyramidal shape with the central nitrogen element in sp3 hybrid state,
the H–N–H Bond angle in NH3 is 106° 45'. This is due to greater repulsion between the two
bond pairs of electrons.
Ammonia is a compound of nitrogen. NH3 is a colorless , quite poisonous gas with a pungent odor. It dissolves very readily in water with the evolution of heat. In solution ammonia forms ammonium hydroxide NH4OH, and behaves as a weak base.
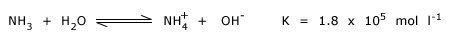
NH3 and NH4OH both react with acids, forming ammonium salts.
NH3 burns in dioxygen with a pale yellow flame.
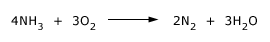
The same reaction occurs in air, but the heat of reaction is insufficient to maintain combustion unless heat is supplied. For example in a gas flame, certain mixtures of NH3/O2 and NH3 /air are explosive.
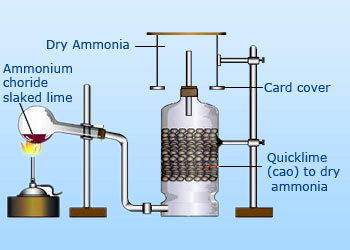 Laboratory preparation of Ammonia
Ammonia gas is lighter than air, necessitating its collection by the
downward displacement of air. Because it is highly soluble in water it
cannot be collected over it. Passing ammonia gas over quicklime (CaO) dries.
Laboratory preparation of Ammonia
Ammonia gas is lighter than air, necessitating its collection by the
downward displacement of air. Because it is highly soluble in water it
cannot be collected over it. Passing ammonia gas over quicklime (CaO) dries.
 Litmus test for Ammonia
Ammonia turns litmus paper blue
Litmus test for Ammonia
Ammonia turns litmus paper blue
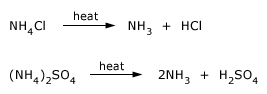
Preparation of ammonia:
Ammonia gas is usually prepared in the laboratory by gently heating
ammonium chloride (NH4Cl) and slaked lime [Ca(OH)2].
2NH4Cl + Ca(OH)2 + Heat  CaCl2 +
2NH4OH
CaCl2 +
2NH4OH  2NH3 (g) +
2H2O
2NH3 (g) +
2H2O
NH3 is prepared in the laboratory by heating an ammonium salt with NaOH. This is a standard test in the laboratory for NH4+ compounds.
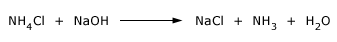
The NH3 evolved may be detected.
- By its characteristic smell.
- By turning moist litmus paper blue.
- By forming dense white clouds of NH4Cl with the stopper from a bottle of HCl
- By forming a yellow–orange–brown precipitate with Nessler's solution.
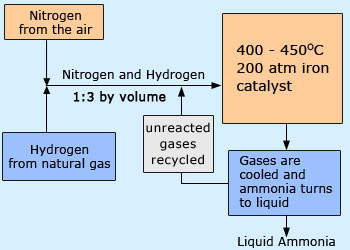 Haber's process
Haber's process
In industries: Most of the ammonia is manufactured synthetically from H2 and N2 by the Haber–Bosch process. About 40 billion pounds of ammonia are manufactured annually in the United States, mostly by the Haber process. The liquid ammonia can be applied directly to the soil as fertilizer. Agricultural use is the largest single application of manufactured NH3.
Process:
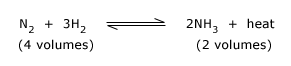
The reaction is reversible, and Le Chatelier's principle suggests that a high pressure and low temperature are required to drive the reaction to the right, and thus form NH3. In practice the conditions used are 200 atmospheres pressure, a temperature of 380–450°C and a iron catalyst. The gas mixture obtained in the process is cooled to condense liquid NH3, and the unchanged mixture of N2 and H2 gases are recycled.
 Ammonium nitrate fertilizer
Ammonium nitrate fertilizer
Ammonium Salts Ammonium salts are all very soluble in water. They all react with NaOH, liberating NH3. The NH4+ ion is tetrahedral.
NH4Cl is well know ammonium salt . NH4Cl is easily purified by sublimation. It can be recovered as a by–product from the Solvay process. It is used in 'dry batteries' of the Leclanche type. It is also used as a flux when tinning or soldering metals, since many metal oxides react with NH4Cl, forming volatile chlorides, thus leaving a clean metal surface.
Ammonium nitrate fertilizer:
NH4NO3 is used in enormous amounts as a nitrogenous fertilizer. It is deliquescent. Because it can cause
explosions it is often mixed with CaCO3 or (NH4)2SO4 to make it safe. It is also
used as an explosive, since on strong heating (above 300°C), or with a detonator, very rapid decomposition occurs.
Smaller amounts of (NH4)2SO4 are also used as a fertilizer.
(NH4)2SO4 is made by passing NH3 and CO2 gases into a slurry of
CaSO4 in water.
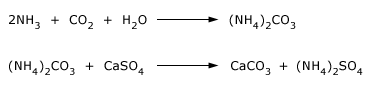
Small amounts of diammonium hydrogen phosphate (NH4)2HPO4 and ammonium dihydrogen phosphate NH4H2PO4 are used as fertilizers. They are also used for fire proofing wood, paper and textiles. NH4ClO4 is used as an oxidizing agent in solid fuel rocket propellants.
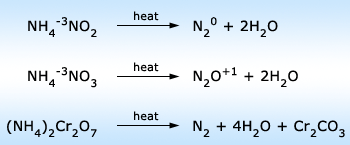 Decomposition reactions of ammonium salts on heating
Decomposition reactions of ammonium salts on heating