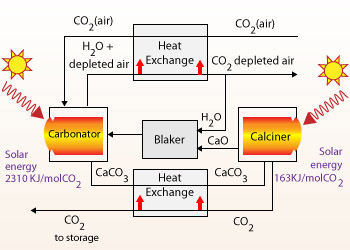
 Calcination
Calcination is the process of subjecting a substance to the action of heat, but without fusion, for the
purpose of causing some change in its physical or chemical constitution usually to drive off the water , CO2 and to
oxidize the substance present in the ore.
Calcination
Calcination is the process of subjecting a substance to the action of heat, but without fusion, for the
purpose of causing some change in its physical or chemical constitution usually to drive off the water , CO2 and to
oxidize the substance present in the ore.
The concentrated Ores are still in their original oxide, carbonate, sulphide or halide forms. To obtain pure metals from these chemicals, it is better convert the metal – compounds into oxides. Oxides of metals can be reduced easily and pure metal can be obtained. To convert the concentrated ores into metal oxides, two processes are used. These processes are calcination (heating in inadequate quantity of air) and roasting the ore (heating in adequate quantity of air).
Calcination:
Carbonate ores are heated in absence of air. The absence of air and heat converts the CO3 into CO2 and O.
The O remains with the metal as metal – oxide. Heating also expels any water content in the ore. In case there are any volatile impurities or gases trapped in the ore, they are also removed by heating.
Example of how calamine ore or zinc carbonate is converted to ZnO by calcination is shown below.

Calcination can be done for ores containing carbonates.
 Roasting is a process of oxidizing zinc sulfide concentrates at high temperatures into an impure zinc oxide,
called "Zinc Calcine". A byproduct of roasting is sulfur dioxide, which is further processed into sulfuric acid, used
as a chemical reagent.
Roasting is a process of oxidizing zinc sulfide concentrates at high temperatures into an impure zinc oxide,
called "Zinc Calcine". A byproduct of roasting is sulfur dioxide, which is further processed into sulfuric acid, used
as a chemical reagent.
Roasting:
Sulphide ores are roasted or heated in plenty of air. The sulphide S changes to sulphur dioxide. The metal reacts with oxygen in
the air to become a metal – oxide. Heating removes gaseous and other volatile impurities.
Example below shows how zinc blende ore (zinc sulphide) is converted to ZnO by roasting
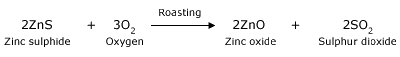
Other types of ores, namely metal – oxide ores and metal – chloride ores remain unaffected by calcination and
roasting processes. On treating with heat, impurities and water are removed from these ores.