 Lanthanoids
Lanthanoids
In the atoms of lanthanides, the nuclear charge increases with atomic number, and the added electrons go to the inner 4f orbitals.
The shielding effect of 4f electrons from the increased nuclear charge is poor. Thus, as the atomic number increases, the effective nuclear charge experienced by each 4f electron increases. This causes a slight reduction in the entire 4f shell. The successive contractions accumulate and the total effect for all the lanthanides is called lanthanoid (or lanthanide) contraction.
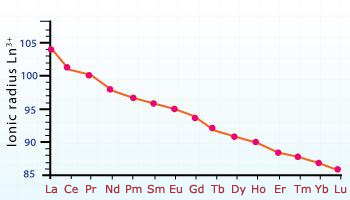 Lanthanides contraction – Ionic radii
Lanthanides contraction – Ionic radii
The lanthanide contraction has a highly significant effect on the relative properties of the elements which precede and follow lanthanides in the periodic table. Some important consequences of lanthanide contraction are:
The radius of La3+ ion, for example, is 22 pm larger than that of Y3+ ion which lies immediately above it in the periodic table. On this basis, if the fourteen lanthanides had not intervened, the radius of Hf4+ should have been greater than that of Zr4+ (which lies immediately above it) by about 20 pm. But, the lanthanide contraction of about the same magnitude almost cancels the expected increase. As a result, Hf4+ and Zr4+ have almost equal radii, being 80 and 81 pm respectively.
It is seen that the normal increase in size from Sc → Y → La disappears after the lanthanides and the pairs of elements such as, Zr–Hf, Nb–Ta, Mo–W, etc., have almost the same size. The properties of these elements are also very similar. It is thus a direct consequence of lanthanide contraction that the elements of the second and third transition series resemble each other much more closely than do the elements of the first and second transition series.
| Name | Symbol | Atomic number | Outer electronic configuration | ||
|---|---|---|---|---|---|
| Cerium | Ce | 58 | 4f1 | 5d1 | 6s2 |
| Praseodymium | Pr | 59 | 4f3 | 5d0 | 6s2 |
| Neodymium | Nd | 60 | 4f4 | 5d0 | 6s2 |
| Promethium | Pm | 61 | 4f5 | 5d0 | 6s2 |
| Samarium | Sm | 62 | 4f6 | 5d0 | 6s2 |
| Europium | Eu | 63 | 4f7 | 5d0 | 6s2 |
| Gadolinium | Gd | 64 | 4f7 | 5d1 | 6s2 |
| Terbium | Tb | 65 | 4f9 | 5d0 | 6s2 |
| Dysprosium | Dy | 66 | 4f10 | 5d0 | 6s2 |
| Holmium | Ho | 67 | 4f11 | 5d0 | 6s2 |
| Erbium | Er | 68 | 4f12 | 5d0 | 6s2 |
| Thulium | Tm | 69 | 4f13 | 5d0 | 6s2 |
| Ytterbium | Yb | 70 | 4f14 | 5d0 | 6s2 |
| Lutetium | Lu | 71 | 4f14 | 5d1 | |
Due to lanthanide contraction, i.e., decrease of ionic size on moving from La3+ to Lu3+, the covalent character in bonding increases in the direction La3+ → Lu3+. As a result, the basic character of the lanthanide hydroxides (M(OH)3) decreases with increase in atomic number.
Thus, La(OH)3 is the most basic, while Lu(OH)3 is the least basic. This aspect has been utilized in the separation of lanthanides from each other.
Due to the lanthanide contraction, the atomic size of the post–lanthanide elements (elements following the last element of the lanthanide series) becomes small. This leads to a better and more compact packing of the atoms in the crystal lattice. Therefore, an appreciable increase in their densities occurs. It is due to this, that the third transition series elements have much higher (almost double) densities as compared to the first and second series elements. As a consequence therefore, the density of Hf and of elements beyond it is higher than that expected on the basis of group characteristics.