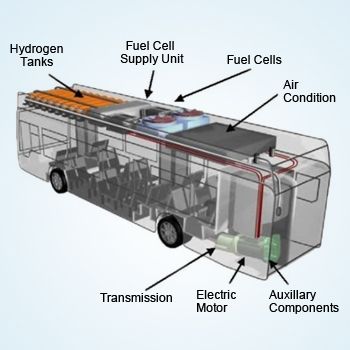
 Hydrogen fuel cell Bus
Hydrogen fuel cell Bus
A fuel cell is a device which convert chemical energy in fuel into electric energy though a chemical reaction, with or without the use of a catalyst. These are combination of a battery and a generator. In fuel cells, usually the hydrogen used as fuel to generate electricity.
Hydrogen is one of two natural elements that combine to make water. Hydrogen is not an energy source, but an energy carrier because it takes a great deal of energy to extract it from water. It is useful as a compact energy source in fuel cells and batteries. Many companies are working hard to develop technologies that can efficiently exploit the potential of hydrogen energy.
Hydrogen (H2) is being aggressively explored as a fuel for passenger vehicles. It can be used in fuel cells to power electric motors or burned in internal combustion engines (ICEs). It is an environmentally friendly fuel that has the potential to dramatically reduce our dependence on imported oil, but several significant challenges must be overcome before it can be widely used.
Fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the only by–products.
 Outline of a Fuel cell
Outline of a Fuel cell
There are many types of fuel cells designed such as Polymer Electrolyte Membrane fuel Cells (PEMFC), Alkaline Fuel cells (AFC), Molten carbonate fuel cells (MCFC), Solid oxides fuel cells (SOFC) etc., but they all work in similar manner.
A single fuel cell consists of an electrolyte sandwiched between two electrodes, an anode and a cathode. Bipolar plates on either side of the cell help distribute gases and serve as current collectors.
In a Polymer Electrolyte Membrane (PEM) fuel cell, which is widely regarded as the most promising for light–duty transportation, hydrogen gas flows through channels to the anode, where a catalyst causes the hydrogen molecules to separate into protons and electrons. The membrane allows only the protons to pass through it. While the protons are conducted through the membrane to the other side of the cell, the stream of negatively–charged electrons follows an external circuit to the cathode. This flow of electrons is electricity that can be used to do work, such as power a motor.
On the other side of the cell, oxygen gas, typically drawn from the outside air, flows through channels to the cathode. When the electrons return from doing work, they react with oxygen and the hydrogen protons (which have moved through the membrane) at the cathode to form water. This union is an exothermic reaction, generating heat that can be used outside the fuel cell.
 A hydrogen fuel cell Car
A hydrogen fuel cell Car
The power produced by a fuel cell depends on several factors, including the fuel cell type, size, temperature
at which it operates and pressure at which gases are supplied.
A single fuel cell produces approximately 1 volt or less – barely enough electricity for even the smallest applications.
To increase the amount of electricity generated, individual fuel cells are combined in series(a few to hundreds of individual
cells) to form a stack.
This "scalability" makes fuel cells ideal for a wide variety of applications, from laptop computers (50–100 Watts)
to homes (1–5kW), vehicles (50–125 kW) and central power generation (1–200 MW or more). Fuel cell system
generate electricity at an efficiencies upto 60 percent.
Researchers have been working to get hydrogen production at fuel–cell temperature–level with no catalyst use. This is full of promise for vehicles powered by hydrogen and other portable electronic items like dig–cams, medical diagnostic devices, defibrillators, cell phones and notebook computers.