| Element | Fluorine (F) | Chlorine (Cl) | Bromine (Br) | Iodine (I) | |
|---|---|---|---|---|---|
| Atomic radius/nm | 0.071 | 0.099 | 0.144 | 0.071 | |
| Ionic radius/nm | 0.133 | 0.180 | 0.195 | 0.215 | |
| Electro negativity | 4.0 | 3.0 | 2.8 | 2 5 | |
| Electron affinity/kJ mol−1 | −328 | −349 | −325 | −295 | |
| Melting point/°C | −220 | −101 | −7 | 114 | |
| Boiling point/°C | −118 | −35 | 59 | 184 | |
| Density/g cm−3 | −1.11 | 1.56 | 3.12 | 4.93 | |
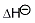 at,[1/2X2(g)
⟶ X (g)]/kJ mol−1 at,[1/2X2(g)
⟶ X (g)]/kJ mol−1 |
79 | 122 | 112 | 107 | |
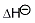 hyd,[X−(g) ⟶ X−(aq)]/kJ mol− 1 hyd,[X−(g) ⟶ X−(aq)]/kJ mol− 1 |
−506 | −364 | −335 | −293 | |
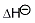 vap,[X2(l) ⟶ X2(g)]/kJ mol− 1 vap,[X2(l) ⟶ X2(g)]/kJ mol− 1 |
3.3 | 10.2 | 15.0 | 20.9 | |
| Valency | H | 1 | 1 | 1 | 1 |
| O | ‐ | 7 | 7 | 7 | |
| Physical state | Gas | Gas | Liquid | Solid | |
| Color (vapor) | Pale yellow | Greenish yellow | Orange | Violet | |
| Color (liquid) | Clear yellow | Amber yellow | Reddish | Shining dark | |
| Comb. with hydrogen | Explosive under all conditions | Explodes in direct sun light | 300°C and platinum catalyst | 300°C and platinum catalyst reversible reaction | |
| Oxidation property | Oxidizes energitically | Oxidizes readily | Oxidizes | Pissle oxidizing action |