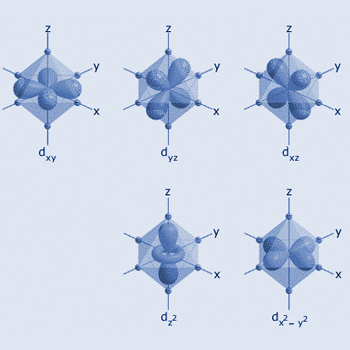
 Shapes of d–orbitals
Shapes of d–orbitals
Crystal field theory(CFT) is a model that describes the breaking of degeneracies of electronic orbital states, usually d or f–orbitals, due to a static electric field produced by a surrounding charge distribution (anion neighbors).
CFT successfully accounts for some magnetic properties, colors, hydration enthalpies, and spinel structures of transition metal complexes, but it does not attempt to describe bonding. CFT was developed by physicists Hans Bethe and John Hasbrouck van Vleck in the 1930's. CFT was subsequently combined with molecular orbital theory to form the more realistic and complex ligand field theory (LFT), which delivers insight into the process of chemical bonding in transition metal complexes.
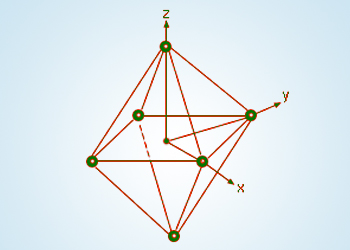 The octahedral geometry
In octahedral symmetry the d–orbitals split into two sets with an energy difference, Δ
oct (the crystal–field splitting parameter) where the dxy, dxz and dyz orbitals
will be lower in energy because group is farther from the ligands than the dz2 and dx2
– y 2 group which will have higher energy, therefore experience less repulsion.
The octahedral geometry
In octahedral symmetry the d–orbitals split into two sets with an energy difference, Δ
oct (the crystal–field splitting parameter) where the dxy, dxz and dyz orbitals
will be lower in energy because group is farther from the ligands than the dz2 and dx2
– y 2 group which will have higher energy, therefore experience less repulsion.The three lower energy orbitals are collectively referred to as t2g, the dxy, dxz and dyz orbitals. The two higher–energy orbitals as eg.dz2 and dx2 – y2
Octahedral Splitting:
The three lower–energy orbitals are collectively referred to as t2g, dxy, dxz and dyz. The two higher–energy orbitals as eg. (These labels are based on the theory of molecular symmetry). dz2, and dx2 – y2.
According to CFT, the interaction between a transition metal and ligands arises from the attraction between the positively charged metal cation and negative charge on the non–bonding electrons of the ligand.
The theory is developed by considering energy changes of the five degenerate d–orbitals upon being surrounded by an array of point charges consisting of the ligands.
As a ligand approaches the metal ion, the electrons from the ligand will be closer to some of the d–orbitals and farther away from others causing a loss of degeneracy.
The electrons in the d–orbitals and those in the ligand repel each other due to repulsion between like charges.
Thus the d–electrons closer to the ligands will have a higher energy than those further away which results in the d–orbitals splitting in energy represented by Δ.
This stability of a complexion is affected by the following factors:
- The nature of the metal ion.
- The metal's oxidation state. A higher oxidation state leads to a larger splitting.
- The arrangement of the ligands around the metal ion.
- The nature of the ligands surrounding the metal ion.
The stronger the effect of the ligands then the greater the difference between the high and low energy d groups. The most common type of complex is octahedral; here six ligands form an octahedron around the metal ion.
Drawback in the Crystal Field Theory:
The crystal field theory could explain the formation, structure, optical and magnetic properties of the coordination compounds
quite satisfactorily.
However, the CFT could not explain the following:
- The existence of covalent bonding in some transition metal complexes.
- The order of ligands in the spectro chemical series.