 Graphite
Graphite
Graphite is found widely distributed in nature, viz., in Siberia, Sri Lanka, USA, Canada. In India, graphite is found in small quantities in Coorg, Chattisgarh, Visakhapatnam and near Patna.
Large quantities of graphite are manufactured from coke or anthracite in an electric furnace.
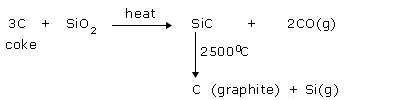
Diamond and graphite are two crystalline allotropes of carbon. Diamond and graphite both are covalent crystals. But, they differ considerably in their properties.
These differences in the properties of diamond and graphite are due to the difference in their structures.
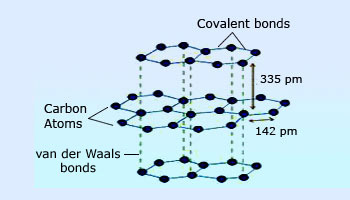 Graphite structure
Graphite structure
Structure of Graphite:
In graphite, the carbon atoms are arranged in regular hexagons in flat parallel layers. Each carbon in these layers is bonded to three others by sp2 covalent bonds. This gives some double bond character to graphite. Each layer is bonded to the adjacent layers by weak van der Waals' forces. As a result, each layer can slide over the other easily. It is because of this structure that graphite is soft, and slippery, and can act as a lubricant. The presence of double bond character (the presence of delocalised p–electrons) makes graphite a good conductor of electricity.
 Graphite in manufacture of lead pencils
Graphite in manufacture of lead pencils
Uses of Graphite
- Graphite is used, as a lubricant at higher temperatures.
- As a refractory material for making crucibles and electrodes for high temperature work.
- In electrotyping and in the manufacture of gramophone records: graphite is used for making the non–conducting (generally wax) surface conducting so that electroplating could be done.
- For manufacturing lead pencils, and stove paints.