 Diamonds
Diamonds
Diamond was discovered for the first time in India. The famous Kohinoor (186 carat) and the Regent or Pitt (studied in Napoleon's state sword, 136.2 carat) were found near Krishna river (South India). Diamonds are chiefly found in the union of South Africa, the Belgian, Congo, Brazil, British Guiana, etc.
 Cullinan diamond
Cullinan diamond
The Cullinan diamond, the largest ever found, weighed 3025. 75 carat (about 600 g) was mined in South Africa in 1905. Diamonds occur in the form of transparent octahedral crystals which usually have curved surfaces and do not shine much in their natural form. They are cut at proper angle so as to give rise to large total internal reflection which lends them the usual shine.
Moissan (1893) prepared the first artificial diamond by heating pure sugar charcoal and iron in a graphite crucible to a temperature of about 3000°C in an electric arc furnace.
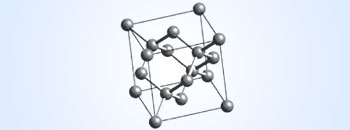 Unit cell structure of Diamond crystal
Unit cell structure of Diamond crystal
- Hybridisation–sp3
- C–C bond length–154 pm
- Crystal type–Cubic crystal
Structure of diamond
In diamond, the carbon atoms are arranged tetrahedrally (sp3 hybridisation of C); each C atom is linked to its neighbours by four single covalent bonds. This leads to a three–dimensional network of covalent bonds. It is because of this, that diamond is very hard, and has high melting and boiling points. Since, all the valence electrons of carbon are used up in forming the covalent bonds, hence diamond does not conduct electricity.
 Tools made of Industrial grade Diamonds
Industrial grade diamonds are used in cutting, grinding, drilling and polishing.
Tools made of Industrial grade Diamonds
Industrial grade diamonds are used in cutting, grinding, drilling and polishing.
Uses of diamonds
- Diamonds are used, as precious decorative stones in jewellery because of their unusual brilliant shine.
- For manufacturing tools for cutting glass and rock–cutting drills because of their hardness.
- In making dies for drawing very thin wires of harder metals. Tungsten wires of thickness 1/6th that of human hair can be drawn using diamond dies.
- For making high precision cutting tools for use in medical field.