 Entropy increases. In general solids are more ordered compare to liquids hence they have less entropy.
A raw egg is in liquid to semisolid state and boiled egg is in solid state.
Entropy increases. In general solids are more ordered compare to liquids hence they have less entropy.
A raw egg is in liquid to semisolid state and boiled egg is in solid state. So when we boil an egg its entropy should decrease but it increases why? This is because when the egg is boiled, denaturation of proteins present in egg yolk takes place.
So the coiled structure starts losing its shape and changes to linear. Hence the entropy increases.
In general all the natural substances in their original state have minimum entropy. When there is any damage or decomposition take place and convert in to any other state of matter (liquid or gas or solid) the entropy increases.
Change in enthalpy
Heat of a reaction (ΔH) of the system in which the energy is gained from the surroundings has a positive
number. It means that ΔH has a positive value for an endothermic reaction as the enthalpy of a system increases.
In an exothermic reaction: H final < H initial and ΔH < 0.
In an endothermic reaction: H final > H initial and ΔH > 0
Entropy is a state function that is often erroneously referred to as the 'state of disorder' of a system. Qualitatively, entropy is simply a measure how much the energy of atoms and molecules become more spread out in a process and can be defined in terms of statistical probabilities of a system or in terms of the other thermodynamic quantities. Entropy is also the subject of the Second and Third laws of thermodynamics, which describe the changes in entropy of the universe with respect to the system and surroundings, and the entropy of substances, respectively.
Enthalpy:
Enthalpy is the amount of heat content used or released in a system at constant pressure. Enthalpy is usually expressed as the
change in enthalpy. The change in enthalpy is related to a change in internal energy (U) and a change in the volume (V), which
is multiplied by the constant pressure of the system.
Enthalpy (H) is the sum of the internal energy (U) and the product of pressure and volume (PV) given by the
equation:
H = U + PV
When a process occurs at constant pressure, the heat evolved (either released or absorbed) is equal to the
change in enthalpy. Enthalpy is a state function which depends entirely on the state functions T, P and U. Enthalpy is usually
expressed as the change in enthalpy (ΔH) for a process between initial and final states:
ΔH = ΔU + ΔPV
If temperature and pressure remain constant through the process and the work is limited to
pressure–volume work, then the enthalpy change is given by the equation:
ΔH = ΔU + PΔV
Also at constant pressure the heat flow(q) for the process is equal to the change in enthalpy defined by the
equation:
ΔH = q
By looking at whether q is exothermic or endothermic we can determine a relationship between ΔH and q. If the reaction absorbs heat it is endothermic meaning the reaction consumes heat from the surroundings so q > 0 (positive). Therefore, at constant temperature and pressure, by the equation above, if q is positive then ΔH is also positive. And the same goes for if the reaction releases heat, then it is exothermic, meaning the system gives off heat to its surroundings, so q < 0 (negative). And if q is negative then ΔH will also be negative.
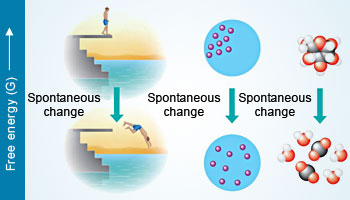 Free energy (G) is a measure of a system's instability: its tendency to change to a more stable state.
Free energy (G) is a measure of a system's instability: its tendency to change to a more stable state.A process that decreases the free energy (ΔG < 0) of the system can proceed spontaneously. Examples include a diver jumping off a platform, diffusion, and exergonic reactions.
| ΔH | ΔS | ΔG | Nature of reactions |
|---|---|---|---|
| −ve | +ve | always −ve | Spontaneous at all temperatures |
| +ve | −ve | always +ve | Non spontaneous at all temperatures |
| −ve | −ve | may be +ve or −ve | Spontaneous if 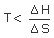 Non spontaneous if 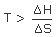 |
| +ve | +ve | may be +ve or −ve | Spontaneous if 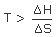 Non spontaneous if 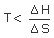 |
Gibbs free energy:
In a system, the chemical process proceeds with certain amount of energy with which a reaction takes place in order to attain
equilibrium. This energy is referred with different names based on the scientist as "affinity" and "Gibbs free
energy". The Gibbs free energy (G), is derived directly from the second law of thermodynamics, which states that any
physical or chemical change must result in an increase in the entropy of the universe. Gibbs free energy is the energy
associated with a chemical reaction that can be used to do work, and is represented by G.
If both T and P are constant, the relationship between the sign of change in free–energy, ΔG and the spontaneity of a reaction is as follows:
- If the change in free energy, ΔG is negative, the reaction is spontaneous in the forward direction.
- If the change in free energy, ΔG is zero, the reaction is at equilibrium.
- If the change in free energy, ΔG is positive, the reaction in the forward direction is non–spontaneous i.e., work must be supplied from the surroundings to make it occur. However, the reverse reaction will be spontaneous.