 Walther Nernst (1864 – 1941)
For the redox reactions in which the reactants and products are not under standard conditions, spontaneity of the reaction can be determined using Nernst equation, first derived by Walther Nernst (1864 – 1941) a German chemist many of the theoretical foundation of electro chemistry. The emf or E generated under non standard condition depends on the concentration of the products and the reactants.
Walther Nernst (1864 – 1941)
For the redox reactions in which the reactants and products are not under standard conditions, spontaneity of the reaction can be determined using Nernst equation, first derived by Walther Nernst (1864 – 1941) a German chemist many of the theoretical foundation of electro chemistry. The emf or E generated under non standard condition depends on the concentration of the products and the reactants. The Nernst equation at 298 K for the reaction between Zn and Cu.

In this reaction n = 2(two electrons are transferred from Zn to Cu2+), and the standard emf is +1.10V. Thus at 298 K the Nernst equation for the reaction between Zn and Cu is —
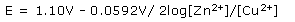
In general, if the concentration of the reactants increase relative to those of the products, the cell reaction becomes more spontaneous and the emf increases. Conversely, if the concentrations of products increase relative to reactants, the emf decreases and the value of Q decreases.
The spontaneity of the reactions which occur at non–standard conditions can be determined using Nernst equation, which in turn depends on the cell emf (E), and concentration of the reactants.
The dependence of the cell emf on concentration can be obtained from the dependence of the free–energy change on concentration. The free–energy change, °G, is related to the standard free–energy change, ΔG°:

The quantity Q is the reaction quotient, which has the form of the equilibrium constant expression except that the concentrations are those that exist in the reaction mixture at a given moment.
Substituting ΔG = –nFE
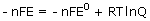
Solving this equation for E gives the Nernst equation:

This equation is customarily expressed in terms of common logarithms:
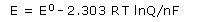
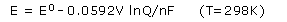
We can use this equation to find the emf produced by a cell under nonstandard conditions or to determine the concentration of a reactant or product by measuring the emf of the cell.