
If a chemical reaction proceeds by more than one step or stage, its overall velocity or rate is limited by the slowest step, the rate–determining step. When we describe the mechanism of a chemical reaction, it is important to identify the rate–determining step and to determine its "molecularity". The molecularity of a reaction is defined as the number of molecules or ions that participate in the rate determining step. A mechanism in which two reacting species combine in the transition state of the rate–determining step is called bimolecular. If a single species makes up the transition state, the reaction would be called uni–molecular. The relatively improbable case of three independent species coming together in the transition state would be called termolecular.
 Dissolving sugar is a physical reaction. When a table spoon of sugar added to the pond water the
concentration of sugar in pond water is negligible
Dissolving sugar is a physical reaction. When a table spoon of sugar added to the pond water the
concentration of sugar in pond water is negligible
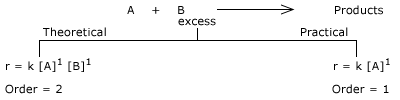
Pseudo First order Reaction:
Suppose, if a table spoon of sugar is added to a glass of water it tastes sweet. If the same amount of sugar is added to pond of
water, the sugar dissolves but the taste of pond water does not change. Like that, if the concentration of one of the reactant
is taken in excess (e.g: solvolysis reactions), change in its concentration is neglected during the course of the reaction. So,
rate of pseudo first order reaction does not depend upon concentration of that reactant and the order of reaction with respect
to that reactant is zero. So the actual molecularity is two but the order is one, such reactions are called as Pseudo first order
reactions.
E.g: Hydrolysis of ester and Inversion of sugar in presence of H+. Here, water is the solvent which is taken in large amount so change in concentration of water is neglected.