The solubility product of magnesium hydroxide Mg(OH)2 at 25° C is 1.4 × 10−11. Calculate the solubility of magnesium hydroxide in grams per litre? (Mg = 24, O = 16, H = 1)
Solution: The solubility equilibrium in this case will be

Let the solubility of Mg(OH)2 be x mol dm−3
∴ Concentration of Mg2+ = x mol dm−3
Now, for each Mg2+ ion, two OH− ions are produced.
∴ Concentration of OH− ions = 2x mol dm−3.
Ksp = [Mg2+][OH−]2
∴ x (2x)2 = 1.2 × 10−11 or
4x3 = 1.2 × 10−11 or
x = 1.44 × 10−4 mol dm−3
∴ Solubility of Mg(OH)2 = 1.44 × 10−4 mol dm−3
= 1.44 × 10−4 mol dm−3 × 58 g mol−1
The solubility product constant (Ksp), of a sparingly soluble salt is defined as the product of the molar concentrations of the ions in a saturated solution of the sparingly soluble salt, each raised to the power equal to the stoichiometric coefficient of the species, in the balanced chemical equation.
To determine the solubility product a variety of experiments have been performed. One of the simplest method is to determine the amount of salt dissolved in 1 liter of water.
For example: For solid MgF2
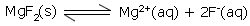
The solubility product constant Ksp expression is –
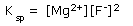
In general, the Ksp values are very small. Such numbers are expressed in terms of ten raised to certain negative powers. For convenience, the solubility product constants (Ksp) can be expressed as p Ksp described by, pKsp = − log Ksp.
Thus, the solubility product, Ksp, of a substance can be calculated if its solubility is known. Since the solubility of a substance changes with temperature, Ksp also changes with temperature.
Applications of Solubility Product:
Knowledge of solubility product is very useful to determine whether precipitates will be obtained or not by the addition of more amount of solute to solution.