 Effect of Temperature on equilibrium
In the above Exothermic reaction the color intensity of products is more when compared to reactants. When we increase the temperature the forward reaction is more favorable. Hence the reaction at room temperature (normal water) gives bright color compare to reaction at below room temperature (ice water)
Effect of Temperature on equilibrium
In the above Exothermic reaction the color intensity of products is more when compared to reactants. When we increase the temperature the forward reaction is more favorable. Hence the reaction at room temperature (normal water) gives bright color compare to reaction at below room temperature (ice water)
In 1888, a French Chemist, Le Chatelier stated a qualitative rule, which enables one to predict the effect of temperature, pressure and concentration on equilibrium of a chemical reaction.
If a chemical system at equilibrium experiences a change in concentration, temperature, volume or partial pressure, then the equilibrium shifts to counteract the imposed change and a new equilibrium is established.
This helps the chemist to predict the favorable conditions for carrying out the reaction.
Effect of temperature: If forward reaction is exothermic, the backward reaction is endothermic. An exothermic reaction is favored by decreasing the temperature but at very low temperature the rate may become very low. So, an optimum temperature is achieved.
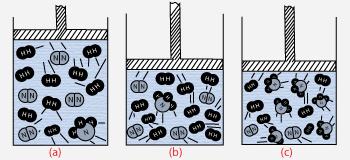 Effect of Pressure on equilibrium
In the above reaction, number of volumes of product (NH3) is less than the number volumes of reactants (N2 and H2). Hence increase in pressure favors the forward reaction.
Effect of Pressure on equilibrium
In the above reaction, number of volumes of product (NH3) is less than the number volumes of reactants (N2 and H2). Hence increase in pressure favors the forward reaction.
Effect of pressure: This is valid for only gaseous phase reactions.
At constant temperature, a reaction, which is accompanied by a decrease in volume, will be favored by increasing the pressure.
For example
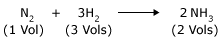
At constant temperature, the reverse reaction, which is accompanied by an increase in volume, will be favored by lowering of the pressure.
Effect of concentration: Yield of the product increases by increasing the concentration of any one of the reacting substances. Similarly the decomposition of the product is favored by increasing the concentration of anyone of the products.
 Effect of Temperature and Pressure on equilibrium concentration of Ammonia
Effect of Temperature and Pressure on equilibrium concentration of Ammonia
Application of Le Chatelier's principle for the Synthesis of ammonia (Haber's process)
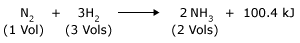
Effect of Temperature: This is an exothermic reaction, hence at low temperature yield of NH3 will be more, but the rate of formation of NH3 is very low at low temperature. Hence, optimum temperature consistent with the workable reaction velocity is necessary (it is 723K in Haber process).
Further, a catalyst (finely chopped iron containing molybdenum) is used to fasten the rate of reaction.
Effect of Pressure: Formation of ammonia takes place with decrease in volume (4 volumes of reactants to 2 volumes of products). Hence increase in pressure favors forward reaction and gives more yield of NH3.
Effect of Concentration: The increase in concentration of H2 instead of N2 has a greater effect in shifting the equilibrium to the right i.e. in producing more amount of NH3.