Relationship between Kc and Kp
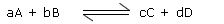
Consider the following reversible reaction
The equilibrium constant for the reaction is expressed in terms of the concentration (mol/litre) may be expressed as
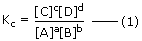
If the equilibrium involves gaseous species, then concentrations must be expressed in terms of partial pressure of gaseous substance. The equilibrium constant in terms of partial pressures may be given as
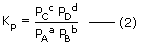
Where PA, PB, PC and PD represent the partial pressures of A, B, C, D respectively. If gases are assumed to be ideal, then according to ideal gas equation.

But we know 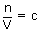 where c = concentration of gas.
where c = concentration of gas.
Hence the equation can be written as
P = cRT
If C is in mole dm−3 and P is in atm, then R = 0.0831 atm dm3 mol−1K−1
Let us suppose consider a general reaction:

The equilibrium constant will be given as
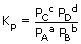
Hence PA = [A]RT where [A] is molar concentration of A
Similarly, PB = [B]RT, PC = [C]RT, PD = [D]RT
Where [B] [C] and [D] are molar concentration of B, C, and D respectively.
Substituting these values in expression for Kp i.e., in equation (2)
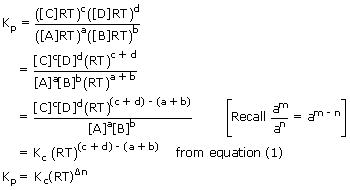
Where Δn = (c + d) − (a + b) i.e., [number of moles of gaseous products] − [number of moles of gaseous reactants] in a balanced chemical reaction
Hence relation between Kp and Kc is given as Kp = Kc (RT)Δn
The ratio of the concentrations of the products and reactants raised to the power of their stoichiometric coefficients, in the equilibrium state is known as Equilibrium Constant.
a A + b B  c C + d D
c C + d D
at equilibrium, equilibrium constant can be written as,
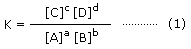
In the above expression, square bracket denotes molar concentration, i.e. concentration in mol/L as mentioned earlier. The expression (1) is also known as Law of Chemical Equilibrium. At a particular temperature, equilibrium constant has a definite value. When we express concentration in mol L−1, equilibrium constant is denoted by Kc.
Equilibrium constant expression for reaction between nitrogen and hydrogen
N2 (g) + 3 H2 (g)  2 NH3 (g) + 92 KJ
2 NH3 (g) + 92 KJ
At equilibrium,
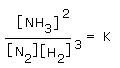
Here, concentration of product (ammonia) occurs in numerator, and those of reactants (hydrogen and nitrogen) occur in denominator. Each concentration term, [NH3], or [N2], or [H2], is raised to a power equal to stoichiometric coefficient in the balanced equation. K is called equilibrium constant.
Equilibrium constant for reactions involving gases: Kp
For gases it is often more convenient to use partial pressures in the equilibrium expression, since the partial pressure of a gas in a mixture of gases is proportional to its mole fraction, which is a measure of its concentration. Therefore, the equilibrium constant for a reaction involving gases is represented by Kp, where K is the equilibrium constant and the subscript P is the partial pressure of the gaseous reactants and product undergoing reaction.
For a general equation:

Therefore, the equilibrium constant in terms of partial pressures, Kp is –
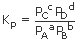
i.e., Kp is equal to the partial pressure of the products divided by the partial pressure of reactants. All calculations and procedures are exactly done in the same manner as Kc.
For example: The reactions that are entirely in gas phase such as –
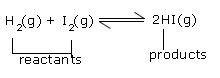
Therefore, the equilibrium constant in terms of partial pressures, Kp is –
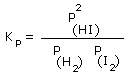
Where P(HI)2, represent the partial pressure of products and PH2 PI2, represent the partial pressure of reactants. It should be noted that, during the calculation of the equilibrium constant in terms of partial pressures, Kp ; SI units of pressure, the Pascal should be used to express the pressure.