Some specific examples of the molar mass relationships
1.1 mol HCl = 36.46 grams of HCl.
2.1 mol CaO = 56.08 grams of CaO.
3.1 mol FeCl3 = 162.20 grams of FeCl3
4.1 mol KCl = 74.55 grams of KCl.
Rounded off molar masses
1.1 mol HCl = 36.5 grams of HCl.
2.1 mol CaO = 56.1 grams of CaO.
3.1 mol FeCl3 = 162.2 grams of FeCl3.
4.1 mol KCl = 74.6 grams of KCl.
Molar Mass (M) is a physical property of a given substance (chemical element or chemical compound), namely it's mass per amount of substance. Relative atomic mass has no units and simply indicates the mass of one element as compared to that of another. One mole of an element is equal to the gram–atomic mass of that element:
1 mole of an element = gram atomic mass of that element
For most calculations, masses may be rounded off to the number of significant figures needed for the calculation.
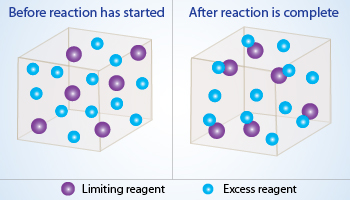 Violet balls being low in number limits the reaction where as excess blue balls are left unreacted making violet balls the limiting reagent.
Violet balls being low in number limits the reaction where as excess blue balls are left unreacted making violet balls the limiting reagent.
Limiting – Reactant Calculations:
Limiting reactant is a reactant in a chemical reaction that limits the amount of product that can be formed. The reaction will stop when all of the limiting reactant is consumed. The amount of the limiting reactant determines how much of the other reactant react and how much of each product is formed. When the amounts of two or more reactants are given, special procedures for limiting–reactant calculations must be used.
Example:
Identify the limiting reactant when 1.22 g of O2 reacts with 1.05g of H2 to produce H2O
Sol : Reaction of H2 with O2 produces H2O
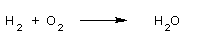
Now balance the reaction
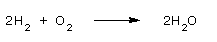
2 moles of H2 reacts with 1 mole of O2 that means 4g (2moles of H2 = 4g) of H2 reacts with 1 mole of O2 (16 grams) to form 36 g (2 × 18 = 36) of water.
Now calculate the moles present in reactants
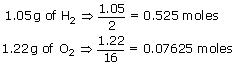
0.525 moles of H2 needs 0.2625 moles of O2. Here we have 0.07625 moles of O2 only. Hence the reaction is controlled by oxygen as it has less number of moles to react with H2. Hence O2 is the limiting reagent.
Worked out example
1. What is the theoretical yield of Fe2S3 that can be formed from 100g of FeCl3 and 50g of H2S ?
Sol: To calculate the mass of Fe2S3
Now, use the 100g of FeCl3
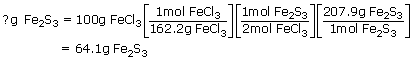
2. When 100g of FeCl3 (Ferric chloride) is reacted with 50.0g of H2S
(Hydrogen sulphide) how many grams of hydrogen chloride are formed?
Sol: 100gm of FeCl3 given in the problem is used as the starting point for
the calculation:
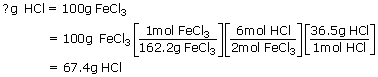
With this calculation we have determined the masses of all reactants and products
in the chemical equation. Listing the masses of the substances before the law of
conservation of matter is obeyed.
Reaction:

| Start | 100g | 0g | 0g | 0g |
| End | 0g | 18.5g | 64.0g | 67.5g |
Determining the theoretical yield:
Theoretical yield is basically the amount of products that are formed in a chemical reaction, when none of the reactants are wasted and the entire reaction is completed. Such reactants that react completely are called the limiting reagents of the reaction. It is based on the stoichiometry of the reaction (i.e., the relation between the quantities of substances that take part in a reaction or form a compound, [typically a ratio of whole integers] and ideal conditions in which starting material is completely exhausted, undesired side reactions or reverse reactions do not occur, and there are no losses in the entire work–up procedure.
The amount of product actually formed by the reaction is the actual yield, which is generally less than the theoretical yield. Therefore, the efficiency of the reaction which is basically the ratio of actual yield to theoretical yield can be expressed as:
Yield (in %) = (actual yield (grams) / theoretical yield (grams)) × 100