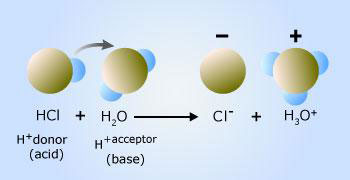
 HCl donates a proton to the water molecule results in a hydronium ion
HCl donates a proton to the water molecule results in a hydronium ion
Hydrogen chloride donates a hydrogen ion to one of the nonbonding electron pairs on a water molecule, resulting in a third hydrogen bonded to the oxygen.
In this case, hydrogen chloride behaves as an acid (proton donor) and water behaves as a base (proton acceptor). The products of this reaction are a chloride ion and a hydronium ion, H3O+, which as show in the Figure, is a water molecule with an extra proton. When added to water, ammonia behaves as a base by accepting a hydrogen ion from water which, in this case, behaves as an acid. This reaction results in the formation of an ammonium ion and a hydroxide ion, which, as shown in the figure, is a water molecule without the nucleus of one of the hydrogen atoms.
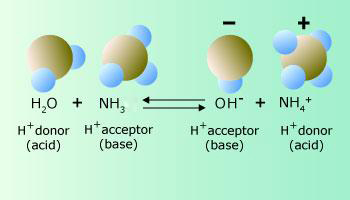 Water molecule donates a proton to the ammonia results in ammonium ion
Water molecule donates a proton to the ammonia results in ammonium ion
The products of an acid – base reaction can also behave as acids or bases. An ammonium ion, for example, may donate a hydrogen ion back to a hydroxide ion to reform ammonia and water. Forward and reverse acid – base reactions proceed simultaneously and can therefore be represented as occurring at the same time by using two oppositely facing arrows. When the equation is viewed from left to right, the ammonia behaves as a base because it accepts a hydrogen ion from the water, which therefore acts as an acid. Viewed in the reverse direction, the equation shows that the ammonium ion behaves as an acid because it donates a hydrogen ion to the hydroxide ion, which therefore behaves as a base.
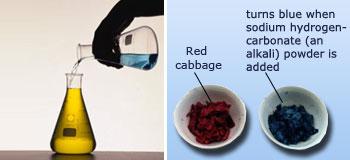 Changing colors by adding chemicals, Vegetable dyes
Changing colors by adding chemicals, Vegetable dyes
Salt is the ionic product of an acid – base reaction. In the language of chemistry, however, salt is a general term meaning any ionic compound formed from the reaction between an acid and a base. Hydrogen chloride and sodium hydroxide, for example, react to produce the salt sodium chloride and water:

Salts can also be obtained when a metal reacts with suitable acid.