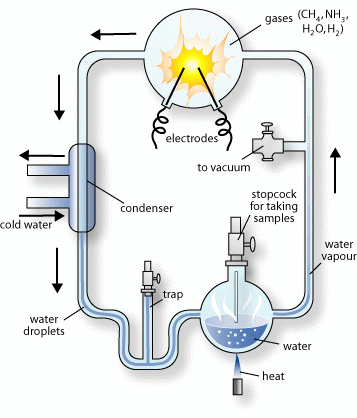
 The experiment did result in the synthesized organic compounds from inorganic precursors.
The experiment did result in the synthesized organic compounds from inorganic precursors.
The Miller–Urey experiment was an experiment that simulated the conditions thought at the time to be present on the early Earth, and tested for the occurrence of chemical origins of life.
Miller–Urey experiment, a mixture of gases (methane, ammonia, hydrogen, and water vapor) representing the primitive atmosphere was subjected to an electrical discharge. The compounds formed were then condensed and accumulated. The solution was found to contain a variety of organic compounds, including aldehydes, carboxylic acid, and amino acids. Thereby, Miller and Urey affirmed that organic compounds could arise in abiotic conditions similar to those that existed on early Earth.
 CO2,N2, H2S, and SO2 are the gaseous molecules released in
Miller–Urey experiment.
CO2,N2, H2S, and SO2 are the gaseous molecules released in
Miller–Urey experiment.
The Miller–Urey experiment has often been repeated, and similar set–ups have generated various organic compounds (including ATP when phosphate is added to the initial slurry). However, there are still various aspects about abiotic synthesis of organic compounds that stimulate much debate. For instance, many scientists feel that the essential ingredients for these early chemical reactions could have come from deep–sea vents in Earth's oceans or from submerged volcanoes, rather than from the early atmosphere. Also, the first cells might have been autotrophs, using inorganic sulfur and iron compounds to generate their ATP, rather than consuming resources heterotrophically.
Ultimately, laboratory simulations are useful for demonstrating what is theoretically possible, yet they cannot ever prove exactly how life originated on Earth. That is a matter that will likely remain open to speculation.
The Miller–Urey experiments demonstrated how organic monomers could have been formed. However, these monomers were present in such dilute concentrations that it is unlikely they could have joined to form polymers. Scientists have proposed that certain types of clay might have catalyzed the polymerization of organic monomers, even in dilute amounts.