 Oxidative Phosphorylation Oxidative phosphorylation is the process in which ATP is formed as a result of the transfer of electrons from NADH or FADH2 to O2 by a series of electron carriers.
Oxidative Phosphorylation Oxidative phosphorylation is the process in which ATP is formed as a result of the transfer of electrons from NADH or FADH2 to O2 by a series of electron carriers. The NADH and FADH2 formed in glycolysis, fatty acid oxidation, and the citric acid cycle are energy–rich molecules because each contains a pair of electrons having a high transfer potential.
When these electrons are used to reduce molecular oxygen to water, a large amount of free energy is liberated, which can be used to generate ATP.
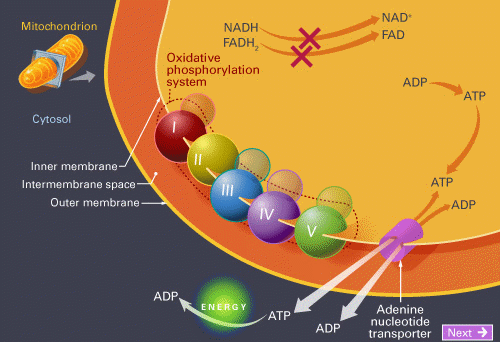
Oxidative phosphorylation is the process in which ATP is formed as a result of the transfer of electrons from NADH or FADH2 to O2 by a series of electron carriers. This process, which takes place in mitochondria, is the major source of ATP in aerobic organisms. For example, oxidative phosphorylation generates 26 of the 30 molecules of ATP that are formed when glucose is completely oxidized to CO2 and H2O.
Thus, Oxidative phosphorylation is conceptually simple and mechanistically complex. The flow of electrons from NADH or FADH2 to O2 through protein complexes located in the mitochondrial inner membrane leads to the pumping of protons out of the mitochondrial matrix. The resulting uneven distribution of protons generates a pH gradient and a transmembrane electrical potential that creates a proton-motive force. ATP is synthesized when protons flow back to the mitochondrial matrix through an enzyme complex. Thus, the oxidation of fuels and the phosphorylation of ADP are coupled by a proton gradient across the inner mitochondrial membrane.