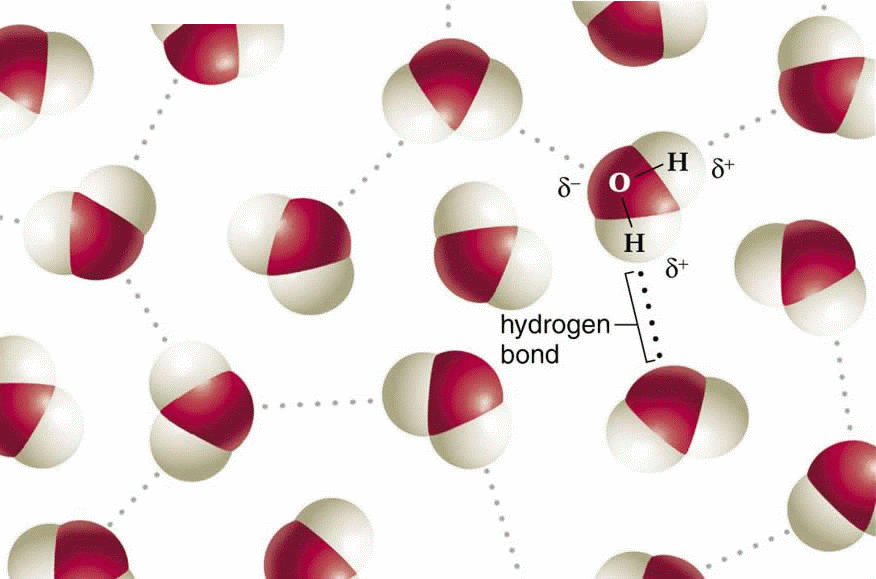
 The water molecule, shaped something like a wide V, is a polar molecule, meaning that opposite ends of the molecule have opposite charges.
The water molecule, shaped something like a wide V, is a polar molecule, meaning that opposite ends of the molecule have opposite charges.
Water is a polar molecule, meaning that it has different electrical properties on opposite ends; specifically, it has two partial positive charges in association with the two H–atoms, and two partial negative charges associated with the oxygen atom.
If we put together, the water molecule is deceptively simple. Its two hydrogen atoms are joined to the oxygen atom by single covalent bonds, because oxygen is more electronegative than hydrogen, the electrons of the polar bonds spend more time closer to the oxygen atom. In other words, the bonds that hold together the atoms in a water molecule are polar covalent bonds. The water molecule, shaped something like a wide V, is a polar molecule, meaning that opposite ends of the molecule have opposite charges: The oxygen region of the molecule has a partial negative charge, and the hydrogens have a partial positive charge. Thus, the anomalous properties of water arise from attractions between these polar molecules. The attraction is electrical; the slightly positive hydrogen of one molecule is attracted to the slightly negative oxygen of a nearby molecule. The two molecules are thus held together by a hydrogen bond.
 CO2 forms a linear molecule that is symmetrical and therefore non–polar.
CO2 forms a linear molecule that is symmetrical and therefore non–polar.
Polar and Non–polar molecules: When two or more atoms form a bond, the entire resulting molecule is either non–polar (symmetrical) or polar (asymmetrical). CO2 forms a linear molecule that is symmetrical and therefore non–polar. It looks like this: O = C = O. H2O is asymmetrical and a highly polar molecule.
Weak attractions exist between non–polar molecules, while strong attractions exist between polar molecules. These attractions are responsible for the physical characteristics of the substance such as solubility. Oils and fats, which are non–polar molecules, will generally dissolve only in non–polar substances. HCl, a polar substance, and NaCl, an ionic one, readily dissolve in a polar substance like water. Other physical characteristics result from the type or strength of the intermolecular bonding. For example, a substance that consists of particles with little or no intermolecular attraction will exist as a gas, while a substance that consists of particles with strong intermolecular attraction will exist as a liquid or solid at room temperature. Substances that are polar will dissolve in water, while substances that are non–polar will not dissolve in water.