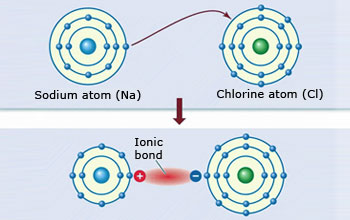
 Ionic bonds results from transfer of electrons
Ionic bonds results from transfer of electrons An ionic bond is the bonding between a non–metal and a metal that occurs when charged atoms (ions) attract after one loses one or more of its electrons,and gives it to the other molecule, for example sodium and chlorine.
This makes the bond stronger and harder to break. In other words, an ionic bond is the electrostatic force of attraction between two oppositely charged ions. The positive ion is called cation, and the negative ion is the anion. It is like the north and south poles of a magnet.
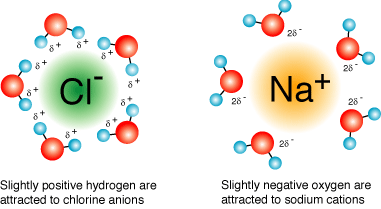 The classic case of ionic bonding
The classic case of ionic bonding Compounds formed by ionic bonds are called ionic compounds, or salts. We know the ionic compound sodium chloride (NaCl) as table salt. Salts are often found in nature as crystals of various sizes and shapes, each an aggregate of vast numbers of cations and anions bonded by their electrical attraction and arranged in a three – dimensional lattice.
A salt crystal does not consist of molecules in the sense that a covalent compound does, because a covalently bonded molecule has a definite size and number of atoms. The formula for an ionic compound, such as NaCl, indicates only the ratio of elements in a crystal of the salt. “NaCl” is not a molecule. Calcium is a metal which is silvery gray in color. Chlorine, on the other hand, is a yellowish – green non–metal.
The transfer of electrons between chlorine and calcium results in the formation of the ionic compound known as calcium chloride. CaCl has several uses in various industries. In construction, it can be used in soil solidification. It enhances dye retention in paper manufacturing. It helps in highway maintenance in the control of ice or snow. And in medicine, it can be used in the treatment of patients with low levels of calcium in the blood.