 Chemical bonds link together atoms to make molecules.
A chemical bond is an attraction between atoms that allows the formation of chemical substances
that contain two or more atoms.
Chemical bonds link together atoms to make molecules.
A chemical bond is an attraction between atoms that allows the formation of chemical substances
that contain two or more atoms.
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms.
Atoms with incomplete valence shells can interact with certain other atoms in such a way that each partner completes its valence shell. The atoms either share or transfer valence electrons. These interactions usually result in atoms staying close together, held by attractions called chemical bonds.
A bond is formed when two atomic nuclei attract the same electrons. Energy is released when a bond is formed, and energy must be supplied to break a bond. Atoms bond to acquire a stable configuration, a completed outer shell.
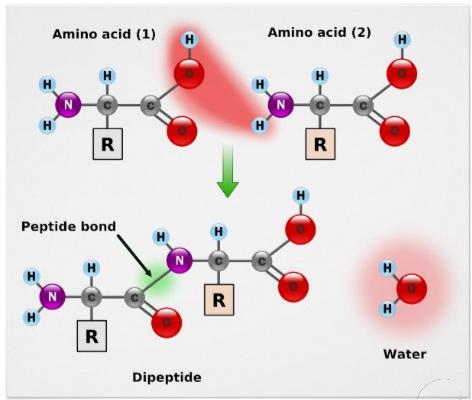 Two aminoacids are joined by a peptide bond.
Two aminoacids are joined by a peptide bond.The atoms in molecules, crystals, metals, diatomic gases and most of the physical environment around us are held together by chemical bonds. Molecular shapes, the three – dimensional arrangements of the atoms that constitute a molecule are determined by the nature of bonds between atoms in a molecule. Molecular geometry determines several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism, and biological activity and hence there is no topic more fundamental to Chemistry than the nature of the chemical bond.
The molecular shapes define all the life processes right from the way a biological “cell” works, the way nerve impulses are communicated and the way immune system works. Genes function when certain nucleic acid molecules fit into specific regions of other nucleic acid. Hydrogen bonding for example plays an important role in determining the three–dimensional structures adopted by proteins and nucleic bases. In these macromolecules, bonding between parts of the same macromolecule causes it to fold into a specific shape, which helps determine the molecule's physiological or biochemical role. The double helical structure of DNA, for example, is due largely to hydrogen bonding between the base pairs, which link one complementary strand to the other and enable replication.